Dissolution Tester
Infrastructure
The system is comprised of the following units:
Jasco DT 810 Dissolution Tester
Jasco LH PV3 Liquid Handler
Jasco FC 812AS Fraction Collector and Sampler
Jasco V 630 Spectrophotometer
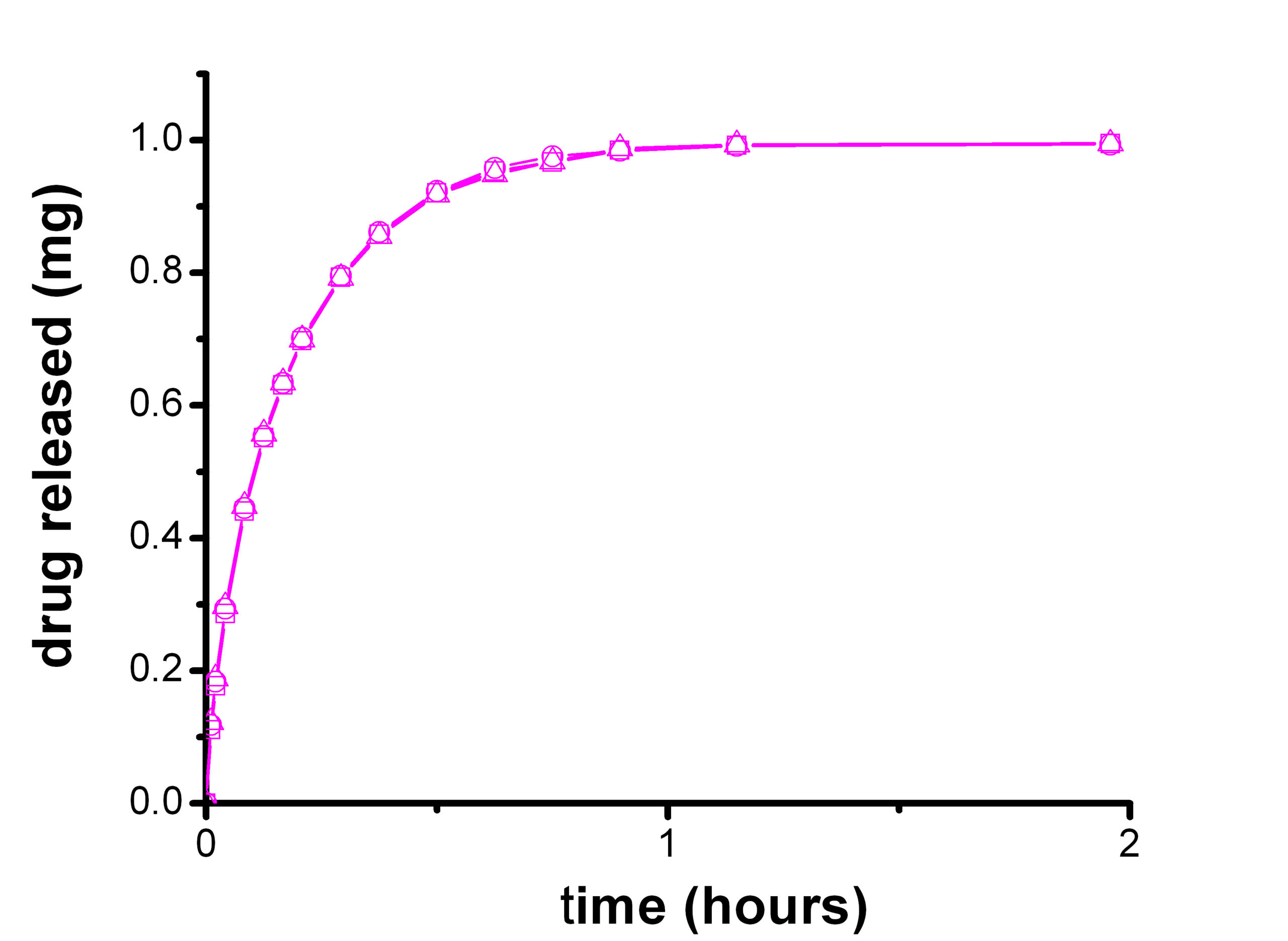
Objective
Dissolution is a test used by the Pharmaceutical industry to characterize the dissolution properties of the active drug, the active drug's release and the dissolution from a dosage formulation. Dissolution testing is used to formulate the drug dosage form and to develop quality control specifications for its manufacturing process. In-vitro dissolution testing is a critical test that has to correlate with in-vivo clinical studies and which could require specific method developments. Dissolution testing is described in many pharmacopoeias, in EP, USP chapters and FDA guidelines.