Cooperative Self-Assembly of Functional Dyes
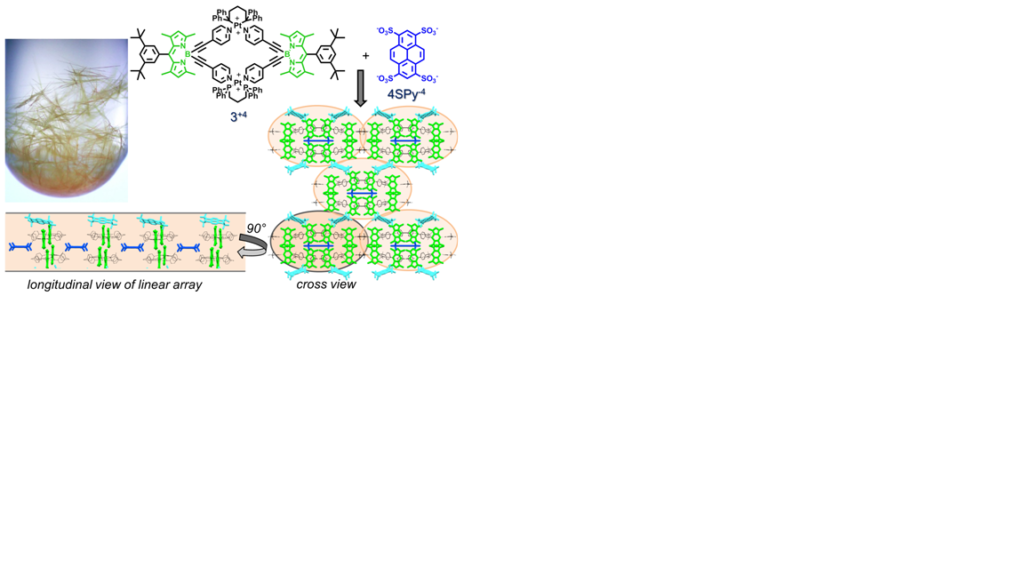
The role of multiple cooperative supramolecular interactions and the working mechanism underlying the formation of sophisticated, well-defined self – assembled architectures, is definitely a challenging and formidable task in understanding the complexity in chemical systems and engineering the properties of advanced materials. In this project we pay attention to the construction of photofunctional supramolecular polymers and networks via the combination of various supramolecular interactions including electrostatic interactions. As an example, taking advantage of the stereodirectionality that can be achieved by both coordination – driven ( Pt+2 – pyridyl) and ionic (SO3- … Pt+2) self-assembly, we combine highly fluorescent coordination self-assembled rhomboid cavitands such as (3+4) with disc-like anionic linkers (4SPy-4) to form nanowires via a self-associative chain reaction.
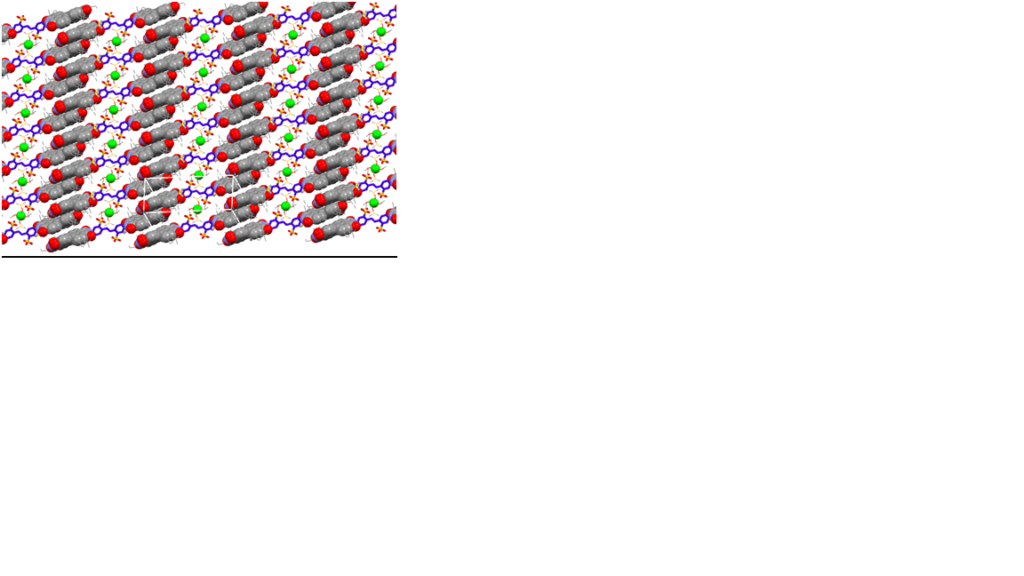
In another part of this activity we provide spectroscopic, structural and mechanistic insights on metal-ion mediated self-assembly of a charged, amphiphilic perylene-bisimide (PBI) dimer S into 2D arrays consisting of parallel columnar PBI stacks with precise spatial arrangement and pattern behavior, using a readily accessible design strategy. The building block (S), a centrosymmetric PBI homodimer bearing a disulfonated trans – stilbene core (Figure 2), was designed to concurrently feature high complexation directionality with strong binding affinity through multiple supramolecular interactions. In solvents that efficiently solvate PBI e.g., chloroform, the zinc ion interacts strongly through electrostatic interactions with the negatively charged core of S, and with the π cloud of the stilbene moiety (cation – π interactions) forming simple 1:1 adducts. In methanol, the findings manifest the efficient formation of well – defined 2D aggregates (Figure 3) with H-type excitonic coupling.