Cyclodextrin Chemistry: designed derivatives for bio- and nano-bio- applications
Cyclodextrins (CDs) are water soluble, nanosized macrocyclic oligomers of glucopyranose. The molecular shape of the macrocycles creates an external hydrophilic surface and an internal hydrophobic cavity, where a plethora of hydrophobic molecules can be encapsulated. The natural members of the family possess a cavity of increased diameter (α-CD < β-CD < γ-CD) thus guest molecules of different sizes can be entrapped. The natural CDs and some of their derivatives are excipients approved by FDA and EMA and are used in pharmaceutical formulations to enhance the aqueous solubility of hydrophobic drugs and regulate their release. A very recent example is the employment of SBECD in the formulation of the antiviral drug remdesivir, in the emergency treatment of COVID-19 patients.
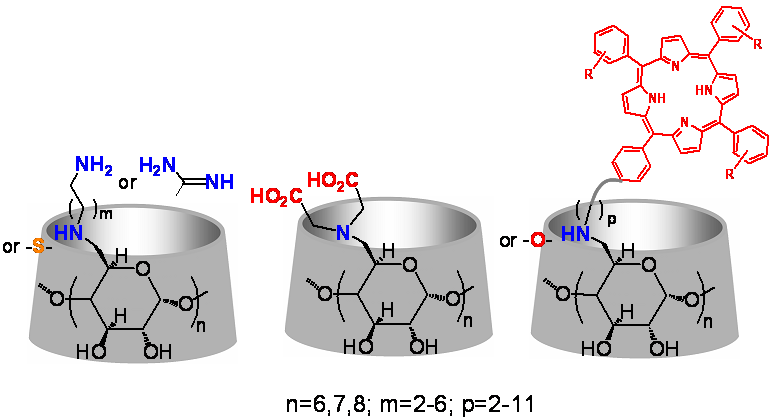
Designed regioselective chemical modifications of CDs can provide derivatives of even higher aqueous solubility, longer cavity, increased encapsulating capacity, strong preference for specific guest molecules and propensity to self-assemble to nanoparticles. Our group specializes in selective functionalization of CDs (Figure 1) and has demonstrated numerous bio- and nano-applications of the synthesized derivatives:
- Cell membrane crossing, drug transport, antibacterial activity: CDs with attached positively charged groups (PCCDs) have been shown to penetrate cell membranes, transport intracellularly molecules and DNA and act as transfection agents. Several of these derivatives are also able to block the channels of pore forming proteins found in many pathogenic bacteria or bind with specific antibiotics and protect them from enzymatic degradation. (Representative Refs 6, 7, 11 )
- Metal binding: CDs with attached negatively charged groups (NCCDs) have been shown to coordinate strongly with metal ions. These derivatives find applications as MRI contrast agents and as strong antibacterial agents towards certain Gram-negative pathogens that restore the activity of β-lactam antibiotics such as carbapenems. (Representative Refs 8,9)
- Photodynamic and chemotoxic drug carriers: CDs modified with photoactive groups such as porphyrins or other photoactive molecules have been shown to act as multimodal molecular systems for transport and release of photosensitizers and anticancer drugs. These entities exert strong photo- and chemo-toxic effects in vitro on various cancer cell lines. (Representative Refs 1-5, 10)
Related references (last 10 years)
- Amphiphilic Chlorin‐β‐cyclodextrin Conjugates in Photo‐Triggered Drug Delivery: The Role of Aggregation. S. Panagiotakis, B. Mavroidi, A. Athanasopoulos, G. Charalambidis, A. G. Coutsolelos, M. Pelecanou, K. Yannakopoulou, ChemPlusChem, 2024, 89, e202300743. DOI: 10.1002/cplu.202300743
- Small anticancer drug release by light: Photochemical internalization of porphyrin-β-cyclodextrin nanoparticles. S. Panagiotakis, B. Mavroidi, A. Athanasopoulos, A.R. Gonçalves, L. Bugnicourt-Moreira, T. Regagnon, N. Boukos, G. Charalambidis, A. G. Coutsolelos, M. Grigalavicius, T. A. Theodossiou, K. Berg, C. Ladavière, M. Pelecanou, K. Yannakopoulou, Carbohydrate Polymers, 2023, 306, 120579. DOI: 10.1016/j.carbpol.2023.120579
- Unsymmetrical, monocarboxyalkyl meso-arylporphyrins in the photokilling of breast cancer cells using permethyl-β-cyclodextrin as sequestrant and cell uptake modulator. S. Panagiotakis, B. Mavroidi, A. Athanasopoulos, G. Charalambidis, A. G. Coutsolelos, M. Paravatou-Petsotas, M. Pelecanou, I. M. Mavridis, K. Yannakopoulou, Carbohydrate Polymers 2022, 275, 118666. doi.org/10.1016/j.carbpol.2021.118666
- A self-locked β-cyclodextrin-rhodamine B spirolactam with photoswitching properties. S. Panagiotakis, E. Saridakis, M. Malanga, I. M Mavridis, K. Yannakopoulou. Chem. Asian J. 2022, 17(2), e202101282 (on-line: 25-11-2021). https://doi.org/10.1002/asia.202101282
- Porphyrinoid-Cyclodexrin Assemblies in Biomedical Research: an Update. I. M. Mavridis, K. Yannakopoulou, J. Med. Chem. 2020, 63, 7, 3391-3424 and references within. https://doi.org/10.1021/acs.jmedchem.9b01069.
- Increased antibiotic efficacy and noninvasive monitoring of Staphylococcus epidermidis biofilms using per-cysteamine-substituted γ-cyclodextrin – a delivery effect validated by fluorescence microscopy. H. Thomsen, M. Agnes, O. Uwangue, L. Persson, M. Mattsson, F. E. Graf, E.-M. Kasimati, K. Yannakopoulou, M. B. Ericson, A. Farewell, Int. J. Pharm. 2020, 587, 119646. DOI:10.1016/j.ijpharm.2020.119646
- Positively charged cyclodextrins as effective molecular transporters of active phosphorylated forms of gemcitabine into cancer cells. V. Rodriguez-Ruiz, A. Maksimenko, G. Salzano, M. Lampropoulou, Y. G. Lazarou, V. Agostoni, R. Gref, K. Yannakopoulou, Scientific Reports 2017, 7: 8353. https://doi.org/10.1038/s41598-017-08727-y
- Iminodiacetic acid-substituted cyclodextrins as potentiators of β-lactam antibiotics, Eur. Patent application EP19386020A·2019-03-27.
- Anionic cyclodextrins as versatile hosts for pharmaceutical nanotechnology: Synthesis, drug delivery, enantioselectivity, contrast agents for MRI. I. M. Mavridis, K. Yannakopoulou, Int. J. Pharm. 2015, 492, 275-290. (Review article). DOI: 10.1016/j.ijpharm.2015.06.004
- Photochemical Internalization of Tamoxifens Transported by a “Trojan-Horse” Nano¬conjugate into Breast-Cancer Cell Lines. T. A. Theodossiou A. R. Goncalves, K. Yannakopoulou, E. Skarpen, K. Berg Angew. Chem. Int. Ed. 2015, 54, 4885-4889. DOI: 10.1002/anie.201500183
- Symmetry requirements for the effective blocking of pore-forming toxins: Comparative study with α-, β-, and γ-cyclodextrin derivatives. K. Yannakopoulou,L. Jicsinszky, C. Aggelidou,N. Mourtzis,T. M. Robinson, A. Yohannes, E. M. Nestorovich, S. Bezrukov, V. A. Karginov, Antimicrob. Agents Chemother. (AAC), 2011, 55, 3594-3597. doi: 10.1128/AAC.01764-10