Structure, intermolecular interactions and properties of host-guest complexes
The LSSC group has long experience in structural studies of cyclodextrin (CD) inclusion complexes in the crystalline state and in aqueous solution. Aspects of complex formation regarding self-assembly in the solid state, strongly influenced by the structure of the guest molecule, complexation variants in solution, guest recognition, enantioselectivity and enantiospecificity issues have been investigated.
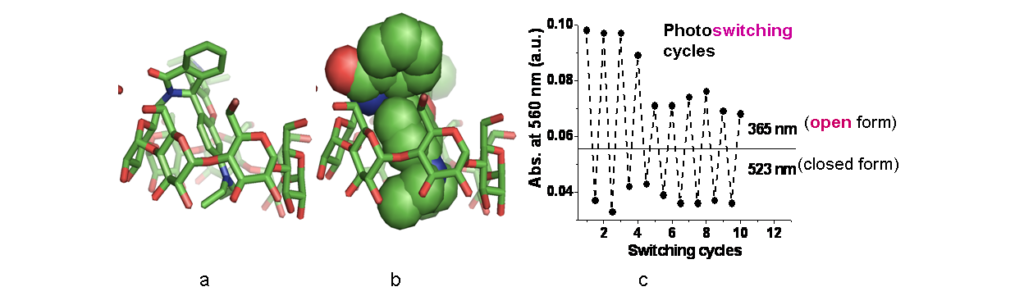
Crystal and solution structures of photoactive molecules enclosed into cyclodextrin hosts: molecular encapsulation alone or combined with covalent attachment controls and regulates the photophysical properties of the guests in aqueous solution, such as fluorescence lifetime, photoswitching (Figure 3), photostability and intracellular localization. (Representative Refs 1, 2, 7).
Crystal and solution structures of bioactive compounds molecularly enclosed into cyclodextrin hosts: comparative studies have been carried out in the solid state and in aqueous solution (mainly by X-ray crystallography and NMR spectroscopy) of CD complexes with guests, such as drugs (acemetacin,triclosan, sulfonylurea anti-diabetic drugs,penicillins, rifampicin, tamoxifen, insect pheromones (dacus and prays oleae sex pheromones), nucleotides, amino acids, as well as various model systems in order to study the factors that drive self-organization,guest recognition, enantioselectivity in binding and guest release. Moreover, Sugammadex (SGM), a γ-cyclodextrin derivative and FDA/EMA approved drug, captures naturally occurring aminosteroid phytotoxins. NMR spectroscopic experiments in solution revealed exceptionally stable inclusion complexes of SGM with the phytotoxin solanidine (SN) and its glycoside α-solanine (ASN) in the pH range of 2–12. Single crystal X-ray analysis of the SGM/SN system (Figure 4) highlighted the importance of the numerous interactions between the host and the steroidal backbone of the guest that prevail in the crystalline state. These phytotoxins have no known antidote to date (Representative Refs 1, 5-7, 9-11).
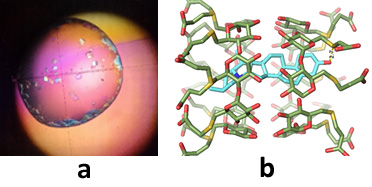
Figure 4. a) Crystals of the Sugammadex/solanidin inclusion complex and b) the crystal (X-ray) structure of the complex.
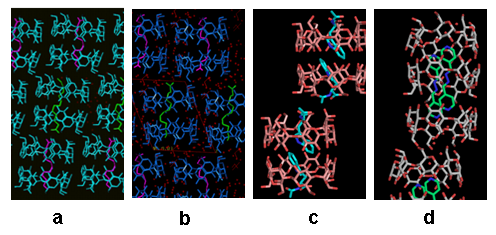
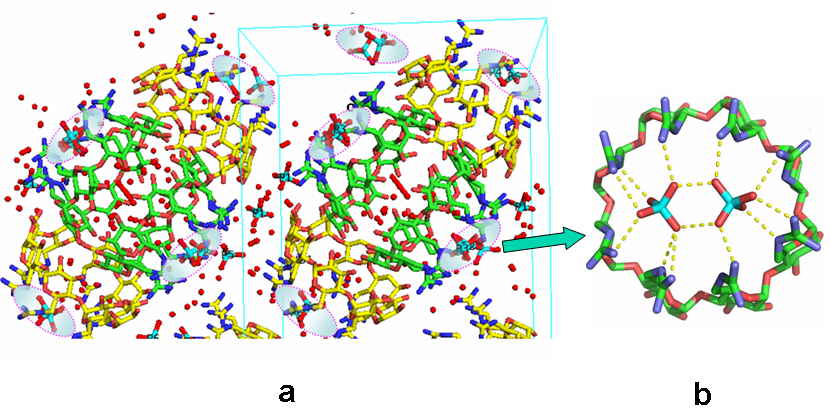
Influence of the guest on the self-assembly of hosts in the crystal: structural studies of β-CD by X-ray analysis have revealed that the majority of complexes form dimers in the crystalline state (Figure 4a,b, c), which pack in four major packing arrangements, however interesting exceptions (trimers) are also observed (Figure 5d). (Representative Refs 2, 3, 4, 11,12). Moreover, as recently found, positive charges on one side of the CD host and presence of suitable counter-ions can result in spectacular 3D organization: octakis-6-guadinidino-γ-cyclodextrin (gguan) hydrochloride in the presence of phosphate anions crystallizes from aqueous solution in the unprecedented form of a superdimer (a dimer within a dimer) that serves as a twin receptor for phosphate dimers. The phosphate dimers are assembled via “anti-eletrostatic” H-bonds (AEHBs) and are stabilized inside each positively charged periphery of the host via a new guanidino-phosphate binding motif (Figure 6). The small (~ 2 nm) gguan-phosphates assembly is pre-organized and stable in the aqueous solution at pH close to neutral, where the guanidino groups are 100% protonated, as found by DLS and NMR experiments (Ref 3).
Related references
- Molecular encapsulation of the aminosteroid phytotoxins solanidine and α-solanine by sugammadex: new insights into atomic-level interactions. E. Kalydi, N. Murvai, E. Saridakis, I. M. Mavridis, M. Malanga, J. Kardos, K. Yannakopoulou, Szabolcs Béni. Carbohydrate Polymers, 2025, 366, 123824. DOI: 10.1016/j.carbpol.2025.123824
- A self-locked β-cyclodextrin-rhodamine B spirolactam with photoswitching properties. S. Panagiotakis, E. Saridakis, M. Malanga, I. M Mavridis, K. Yannakopoulou. Chem. Asian J. 2022, 17(2), e202101282 (on-line: 25-11-2021). https://doi.org/10.1002/asia.202101282
- Unsymmetrical, monocarboxyalkyl meso-arylporphyrins in the photokilling of breast cancer cells using permethyl-β-cyclodextrin as sequestrant and cell uptake modulator. S. Panagiotakis, B. Mavroidi, A. Athanasopoulos, G. Charalambidis, A. G. Coutsolelos, M. Paravatou-Petsotas, M. Pelecanou, I. M. Mavridis, K. Yannakopoulou, Carbohydrate Polymers, 2022, 275, 118666. on-line 21/9/2021; doi.org/10.1016/j.carbpol.2021.118666
- A guanidino-γ-cyclodextrin superdimer generates a twin receptor for phosphate dimers assembled by anti-electrostatic hydrogen bonds. E. Saridakis, E.-M. Kasimati, K. Yannakopoulou, I.M. Mavridis, ChemComm, 2022, in print. https://doi.org/10.1039/D2CC00323F
- Molecular recognition of N-acetyltryptophan enantiomers by β-cyclodextrin. S. D. Chatziefthimiou, M. Inclán, P. Giastas, A. Papakyriakou, K. Yannakopoulou, I. M. Mavridis Beilstein J. Org. Chem. 2017, 13, 1572-1582. https://doi.org/10.3762/bjoc.13.157
- Formation, characterization and pH dependence of rifampicin:heptakis(2,6-di-O-methyl)-β-cyclodextrin complexes. L. Angiolini, M. Agnes, B. Cohen, K. Yannakopoulou, A. Douhal, Int. J. Pharm. 2017, 531, 668-675. DOI: 10.1016/j.ijpharm.2017.06.015
- Designed positively charged cyclodextrin hosts with enhanced binding of penicillins as carriers for the delivery of antibiotics: the case of oxacillin, M. Agnes, A. Thanassoulas, P. Stavropoulos, G. Nounesis, G. Miliotis, V. Miriagou, E. Athanasiou, G. Benkovics, M. Malanga, K. Yannakopoulou, Int. J. Pharm. 2017, 531, 480-491. DOI: 10.1016/j.ijpharm.2017.04.080
- Photochemical Internalization of Tamoxifens Transported by a “Trojan-Horse” Nanoconjugate into Breast-Cancer Cell Lines. T. A. Theodossiou A. R. Goncalves, K. Yannakopoulou, E. Skarpen, K. Berg, Angew. Chem. Int. Ed. 2015, 54, 4885-4889. DOI: 10.1002/anie.201500183
- S-Nitroso-β-Cyclodextrins as Novel Bimodal Carriers: Preparation, Detailed Characterization, Nitric Oxide Release and Molecular Encapsulation. L. Piras, T. A. Theodossiou, M. D. Manouilidou, Y. G. Lazarou, S. Sortino, K. Yannakopoulou, Chem. Asian J. 2013, 8(11), 2768-2778. DOI: 10.1002/asia.201300543
- Cooperative heterodimer formation between per-guadinylated and carboxylated or phoshphyorylated cyclodextrins in DMSO and DMSO-water studied by NMR spectroscopy and Microcalorimetry. K. Fotiadou, A. Thanassoulas, G. Nounesis, K. Yannakopoulou, Supramol. Chem. 2011, 23, 493-500. https://doi.org/10.1080/10610278.2010.550918
- Real time monitoring of nanomolar binding to a cyclodextrin monolayer immobilised on a Si/SiO2/Novolac surface using White Light Reflectance Spectroscopy: The case of triclosan. D. Maffeo, Z. Velkov, K. Misiakos, K. Mergia, A. Paulidou, M. Zavali, I. M. Mavridis, K. Yannakopoulou J. Colloid. Interface Sci. 2011, 358, 369–375. DOI: 10.1016/j.jcis.2011.03.024
- Similar modes of inclusion in complexes of β-cyclodextrin with sulfonylurea hypoglycemic drugs. A. Paulidou, D. Maffeo, K. Yannakopoulou, I. M. Mavridis, Cryst. Eng. Commun. 2010, 12 (2): 517-525 DOI: 10.1039/b908128c.
- Binding of nucleotides and nucleosides to per(6-guanidino-6-deoxy)-cyclodextrins in solution. Ch. Aggelidou, I. M. Mavridis, K. Yannakopoulou Eur. J. Org. Chem. 2009, 2299-2305. https://doi.org/10.1002/ejoc.200900040