Carbon-based Materials for Environmental and Energy Applications
Non-activated high surface area expanded graphite oxide
The preparation of graphene by chemical methods offers the possibility of producing it on a large scale and, at the same time, of controlling its quality. This depends on the properties of (i) the pristine graphite, (ii) the oxidation method used, and (iii) the final reduction of the graphene oxide (GO) to graphene.In the present study some of these factors were investigated. The natural graphite without pretreatment and expandable graphite pretreated in a microwave oven (850 W) were used for the preparation of GO according to a modified Staudenmaier method. After oxidation the produced graphite oxide (GtO) were subsequently subjected to microwave irradiation since during this treatment both exfoliation and reduction occurs. All the types of graphite that were tested had high BET surface areas ranging from 940 to 2490 m2/g. According to cyclic voltammetry (CV), all samples exhibited supercapacitor behavior.
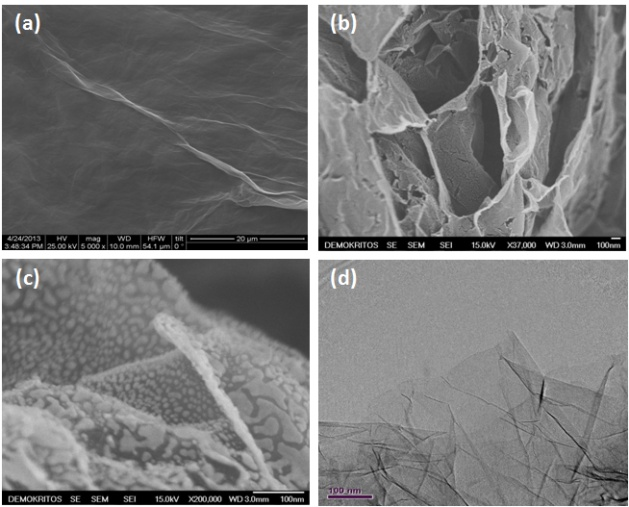
Single-step hydrothermal synthesis of reduced graphene oxide and Fe2O3/reduced graphene oxide compositesHydrothermal process constitutes a facile, one-step, low cost and environmental friendly approach that results in production of freestanding reduced graphene oxide (rGO) sheets as well as intercalated with nanoparticles rGO sheets to prevent restacking. In this study, rGO sheets and their intercalated with Fe2O3 analogues were prepared using this treatment process.
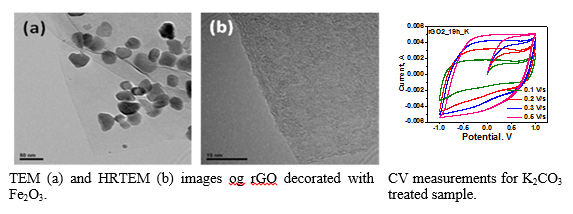
TEM image presents rGO sheets decorated by Fe2O3 nanoparticles (a). In HRTEM image (b), a few layer graphene material is observed supporting our claim about hydrothermal exfoliation to a significant extend. CV measurements indicated that the sample treated with K2CO3 had the best performance in terms of capacitance.
Graphitic materials decorated with Ag nanoparticles
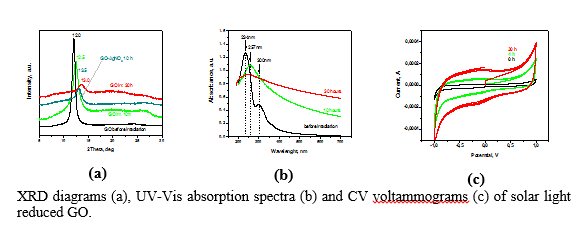
The electrical properties of graphenes can be improved by decoration with metal nanoparticles. The incorporated particles also prevent aggregation and further restacking of graphenes thus acting as both conductor and nanospacer. In our lab the activities were focused on deposition of silver (Ag) on GO sheets.Ag nanoparticles were deposited via chemical reduction of Ag salts using NaBH4 and photo-reduction using solar light irradiation. The shift from GtO prepared by Hummers method to rGO was evidenced by XRD, FT-IR, Raman and XPS analysis. The CV measurements revealed enhancement in the specific capacitance with the increase of the reduction degree. The photo-reduction of GO using solar light that can be easily complemented with simultaneous metal nanoparticles deposition provides a green sustainable route towards preparation of graphene-based heterostructures for applications beyond that of supercapacitors.
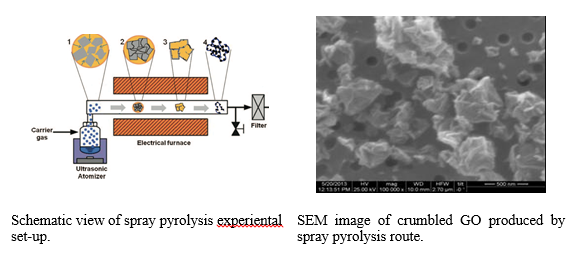
Crumpled graphene oxide by Spray Pyrolysis (SP)
Restacking of graphene/graphene oxide sheets is a serious problem of these materials. To avoid restacking except for chemical routes based on pillaring procedures, the sheets’ crumpling using spray pyrolysis is proposed.
Spray pyrolysis system was constructed consisting of three main parts: (1) an atomizer, which atomizes the precursor solutions and converts them to droplets, (2) a vertical furnace (length: 130 cm, diameter: 5 cm) with controllable temperature until 1200oC, and (3) a filter to collect prepared nanoparticles.
GtO was prepared using Hummer’s synthetic route. Dried GtO powder was added to water at ~1.5 mg/L forming dispersion that was sprayed using an aerosol generator. Passing through a furnace heating zone at 300 degrees, the droplets formed crumpled GO preventing GO sheets from restacking.
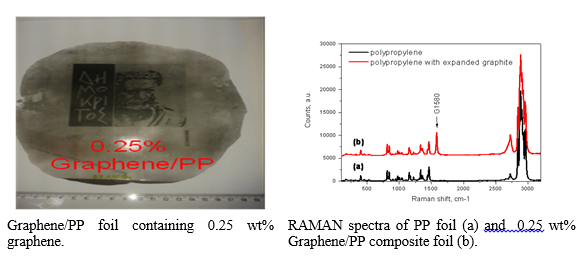
Graphene/polymer composite materials
In order oxygen barrier property and controlled water vapor permeability to be granted to the polymer, microwave exfoliated graphite (GNP) and graphene oxide were introduced in polypropylene (PP) matrix. The quantities, composition and the method of introduction of the graphene component were investigated targeting the mentioned properties, the homogeneity and the mechanical properties of the graphene/PP materials. Although increased oxygen barrier ability was achieved after addition of GNP, the mechanical properties were not improved. To overcome this obstacle, functionalization of graphene is in progress.
Electrochemical deposition of few-layer graphene films on ITO substrates
Electrochemical deposition (ECD) is a process based on the movement of charged particles in suspension toward an oppositely charged electrode and their deposition on it. The EDP of graphene films is possible from GO suspensions due to presence of oxygen-containing groups like epoxides and carboxylic acids on GO surface. These groups endow the GO flakes with hydrophilic properties and therefore contribute to creation of stable aqueous suspensions which is important aspect in ECD. Also, the deprotonation of carboxylic groups in water leads to formation negatively charged moieties that can be deposited on positively charged electrode.
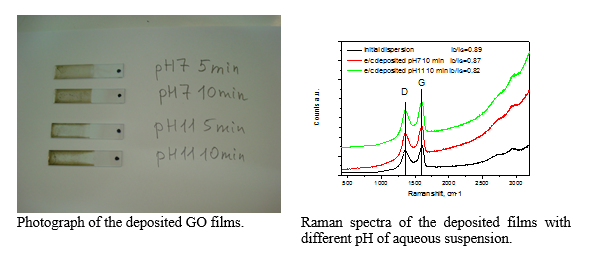
GO films were deposited on ITO substrate under application of +4 V electrical potential and controlling deposition time and pH of the GO (1 mg/mL) aqueous suspensions. The Raman spectra exhibit small decrease in ID/IG ratio with the increase of pH that is attributed to partial simultaneous reduction of GO films during deposition procedure
Nanocarbon materials and polymers for water treatment applications
Nowadays, with fresh water scarcity being one of the most challenging global issues, several desalination technologies have been proposed for sustainable water recovery. Capacitive deionization (CDI) has emerged as an efficient, energy-saving approach for brackish water desalination. CDI technology is based on ion electrosorption at the surface of a pair of electrically charged electrodes, commonly composed of highly porous carbon materials owing to their high specific surface area and specific capacitance.
The object of this project is the development of nanocomposite electrodes consisted of nanocarbon materials combined with conductive polymers for efficient desalination performance. In brief, carbon nanostructures with hierarchical porous structure were successfully manufactured from sucrose and egg white precursors via hydrothermal treatment and ice-templating, respectively. The impact of physical and chemical activation on the porous structure of the final carbonaceous material was investigated. For the physical activation, the samples were heated under high-purity carbon dioxide (CO2) gas flow, while the chemical activation involved mixing of material with potassium hydroxide (KOH) solution and heating under inert gas flow. The activated carbon nanostructures derived from sucrose and egg white were denoted as HTC-A and EWC-A, respectively. Composite materials containing carbon and conductive polymers were developed as the active material of the electrodes for water treatment. The conductive polymer here is polypyrrole, having several advantages such as good electrical conductivity, electroactivity, chemical stability in both acidic and alkaline environments, low cost and tunable morphology. Regarding the synthesis of the one-dimensional polypyrrole, a template was initially developed serving as a binding site for monomer. This template was based on the oxidant (FeCl3) that is necessary for polymerization and in combination with Methyl Orange azo-dye, a tubular template structure was formed. The polymerization process begins when the monomer was added to the aqueous solution.
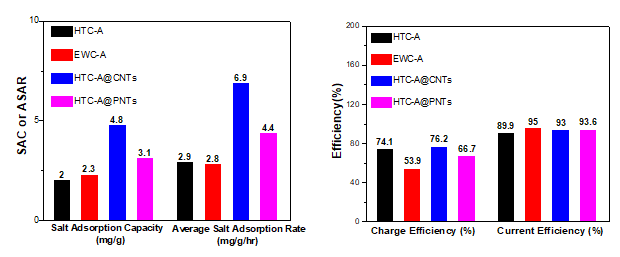
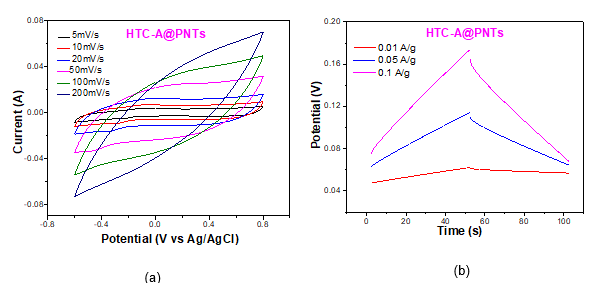
Nanocomposite electrodes were manufactured by mixing the active material with a small quantity of binder and stirring until a homogeneous slurry is formed. The slurry was then deposited on graphite substrates as a coating. Furthermore, hybrid electrodes with carbon nanotubes (HTC-A@CNTs) and polypyrrole nanotubes (HTC-A@PNTs) as additives were also tested to delineate the impact of specific surface area and conductivity of different active materials (Fig. 1) on electrode performance.
The electrochemical performance of the nanocomposite electrodes was evaluated in 1M sodium chloride (NaCl) aqueous solution using a three-electrode configuration with porous carbon, platinum (Pt) sheet and silver/silver chloride (Ag/AgCl) as working, counter and reference electrode, respectively. The specific capacitance of the nanocomposite electrodes was determined by Cyclic Voltammetry (CV) and Galvanostatic Charge-Discharge. (GCD) (Fig.2). It was observed that the one-dimensional nanotubular polypyrrole had notable electrochemical performance. The abundance of active sites in this particular structure compared to the typical globular polypyrrole structure maximizes contact between polymer and both the electrolyte and nanocarbons. Thus, the synergistic effect between the two materials was more intense and moreover the diffusion path of the conductivity carriers within the material was reduced. In addition, the reversibility and the intensity of the redox reactions was enhanced.
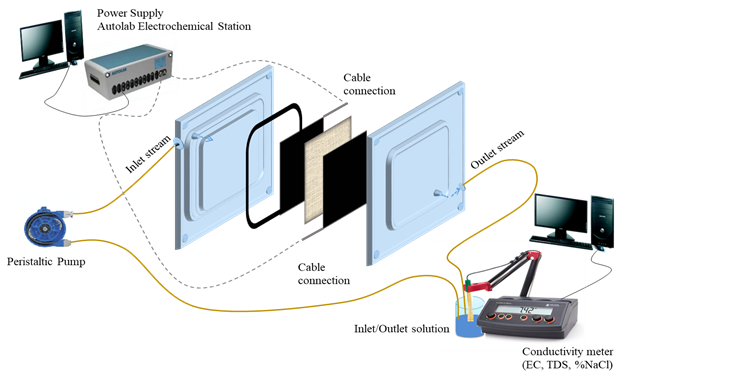
Desalination effectiveness of the nanocomposite electrodes was examined employing a lab-scale CDI cell with synthetic sodium chloride (NaCl) solution. In each CDI experiment, 300ppm NaCl salt solution with 200 mL total volume was circulated between an outside reservoir and the deionization cell using a peristaltic pump at a constant flow rate. The experimental stet-up of process is presented in Fig. 3.
Impressively, a low content of carbon nanotubes (only 1wt %) has resulted in significant increase of salt adsorption capacity leading to enhanced desalination performance of the composite electrodes. The outcome was ascribed to the synergistic effect of both carbons with its upgraded electrical conductivity inherited by CNTs combined with the high specific surface area (1495m2/g) of activated carbon making them attractive for application as electrode materials for capacitive deionization. The fabricated composite electrodes showed an excellent performance with capacitance of 463 mF/cm2 (at 5 mV/s), salt adsorption capacity of 4.8 mg/g and current efficiency of 93% at 1.2 V in 300 ppm NaCl solution. (Fig. 4)