Conversion of CO2 to High Added Value Products
Being one of the major greenhouse gases, carbon dioxide (CO2) is responsible for the global warming due to its constantly increasing emissions from human activities. The CO2 conversion into solid nanocarbons such as graphene, carbon nanotubes (CNTs) or nanofibers (CNFs) is a promising way to address the mentioned pollution problem. The NNML participates in this effort on CO2 mitigation by coordination of the project entitled “NanoCARBONs from GREENhouse gasses of cement industry” (CARBONGREEN) / “ΝανοΑΝΘΡΑΚες από αέρια θερμΟΚΗΠιΟυ ΤσιμεντοβιομηχανίαΣ” (ΑΝΘΡΑΚΟΚΗΠΟΣ) - T1EDK-01729 (MIS 5848538) co‐financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH-CREATE-INNOVATE. The partners are involved in this project are the Greek companies ΤΙΤΑΝ Α.Ε. and MEGARA RESINS S.A. The main activities of the Lab are focused on the development of experimental processes and technology to convert CO2 of cement plants into useful solid nanocarbons and simultaneously to use them for fabrication of composite products with improved properties. For this purpose, the Lab succeeded to develop the CO2 conversion processes based on CO2 metallothermic reaction and electrolysis of molten salts.
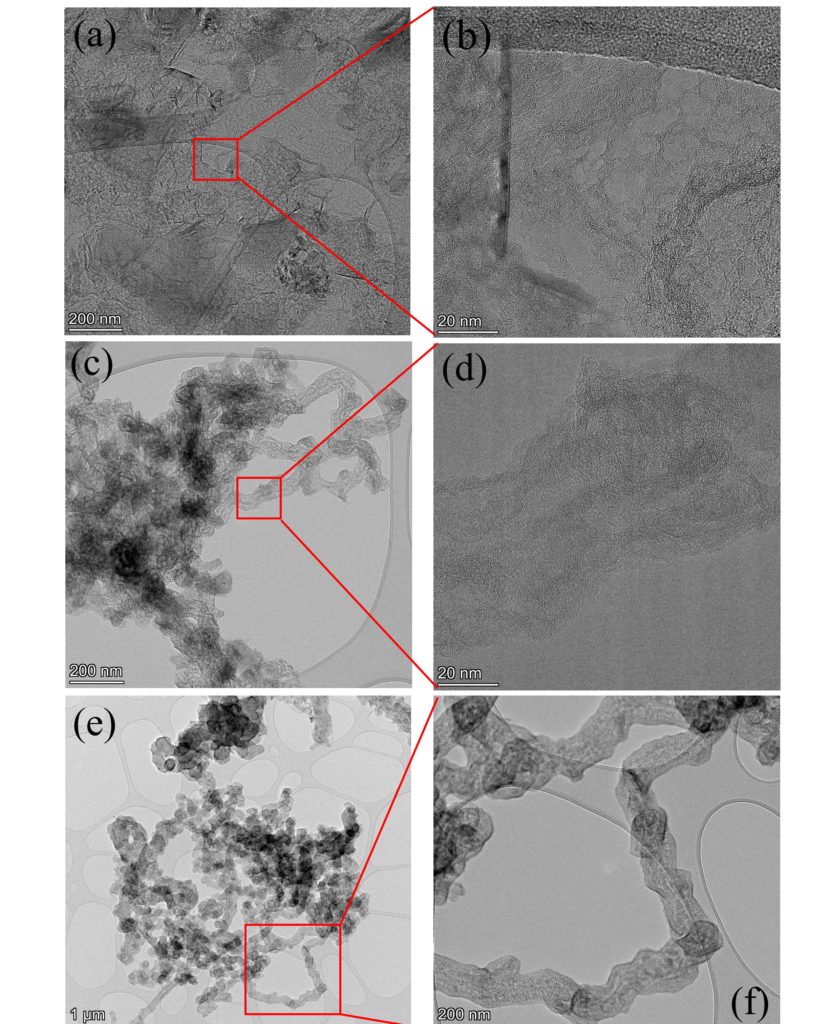
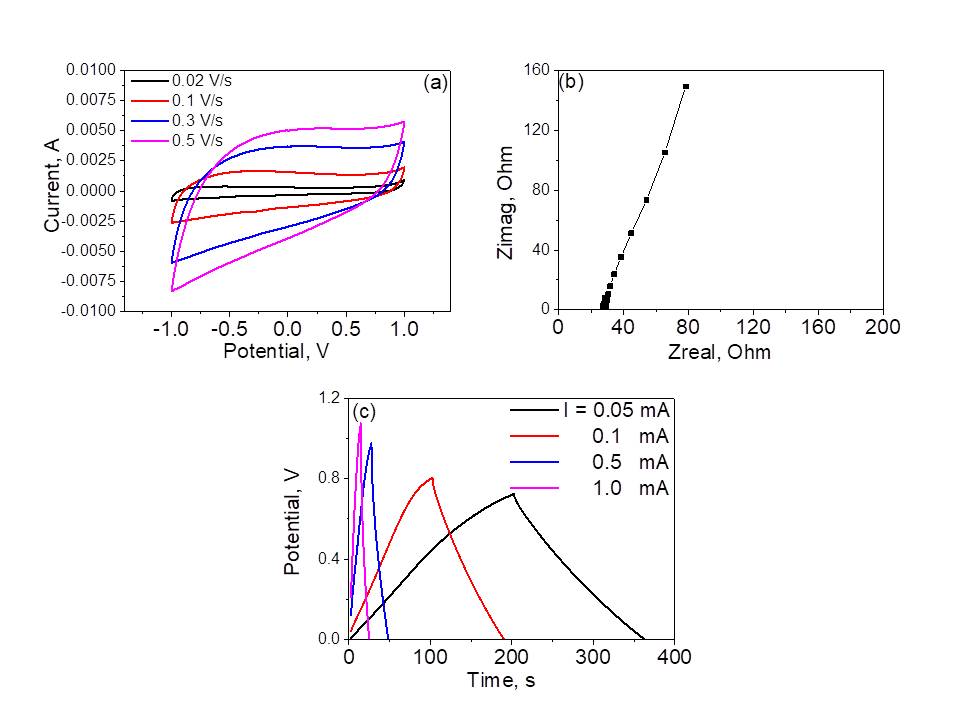
The metallothermic CO2 reduction is performed in a tube furnace under a continuous CO2 flow over a lone Mg reductant or its mixtures with other metals or chemicals. As an example, Fig. 1 demonstrates the nanocarbons produced using only Mg reductant for synthesis at temperature slightly higher that the melting point of Mg. The obtained material is a mixture of the nanostructures with sheet-like, spherical and tubular geometry. It exhibits high crystallinity, low disorder and remarkable electrochemical perfomance (Fig. 2). Thus, the capacitance estimated from the CV loops reaches a value of ~ 407 F/g at scan rate of 0.02 V/s.
The electrolysis of molten salts is carried out in a specially designed vertical furnace. The furnace cover contains several holes for introduction of CO2 and insertion of electrodes. The variation of synthetic conditions, in particular electrolyte composition, electrode material or reaction temperature results in the synthesis of nanocarbons with different morphologies like graphene, carbon nanotubes or nanofibers, etc.
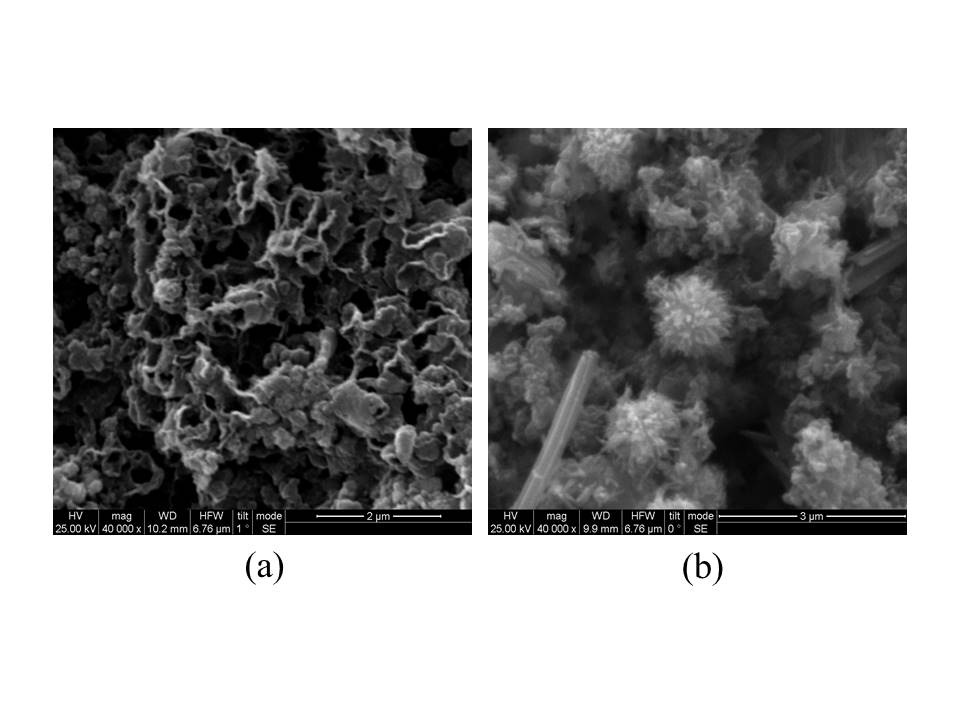
For example, carbon honeycomb nanostructures or short tubes resembling urchin-like agglomerates are formed when Li-K-Na carbonate electrolyte and different anode electrodes were used (Fig. 3).