Photoactive Materials for Environmental and Energy Applications
Morphosynthesis of anatase nanocrystals with exposed {001} facets for environmental applications
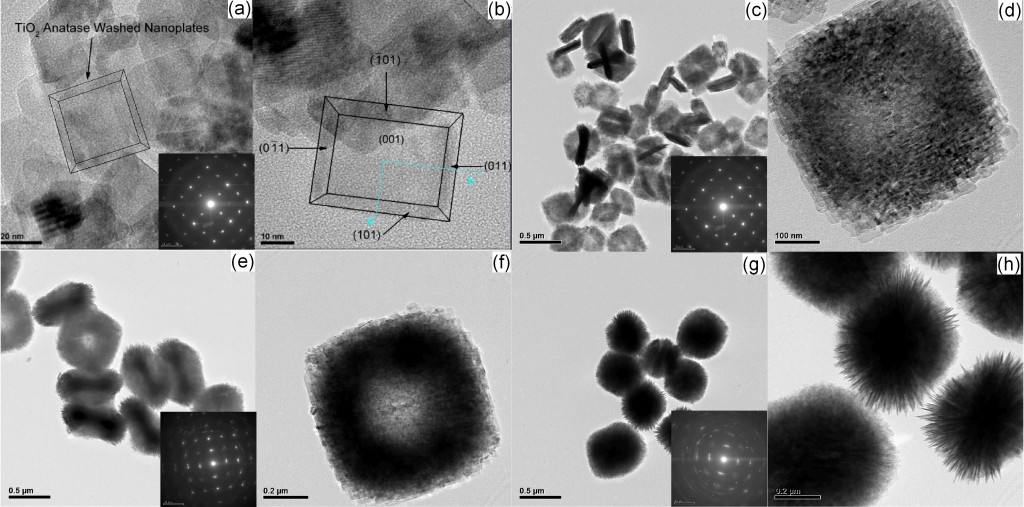
The anatase phase of TiO2 has in most cases higher photocatalytic activity than the other crystalline allotropies. Recently several studies have shown that the (001) surface of anatase is more reactive than the thermodynamically stable (101) surface. This implies that anatase structures with dominant {001} crystal facets will exhibit enhanced photocatalytic properties

In our laboratory, the formation of dominant {001} crystal facets was managed by combining hydrofluoric acid and other non-toxic substances as a capping agent, which facilitates the formation of these crystal facets by lowering their free energy and making them more stable. The anatase structures produced using hydro/solvothermal method are micro/nanoplates and hollow microspheres composed of crystals with {001} dominant facets. The TiO2 samples with mentioned morphologies exhibited higher photonic efficiency in NO oxidation and acetaldehyde decomposition than Evonik Degussa P25, which was used as a reference.
Decoration of TiO2 Anatase Nanoplates with Silver Nanoparticles on the {101} Crystal Facets
Ag nanoparticles were photodeposited on the {101} crystal facets of the TiO2 anatase nanoplates. This was achieved with the photoreduction of AgNO3 in methanol solution in which TiO2 anatase nanoplates were suspended while UVA irradiation was performed. The manipulation of their size was achieved by controlling the UVA light irradiation period of time. Under illumination, electron and hole pairs are created which move to the surface of the nanoplates and are spatially separated. The electrons migrate to the {101} facets while the holes migrate to the {001} facets. As a result, Ag clusters are formed exclusively located on the {101} facets of the nanoplates.
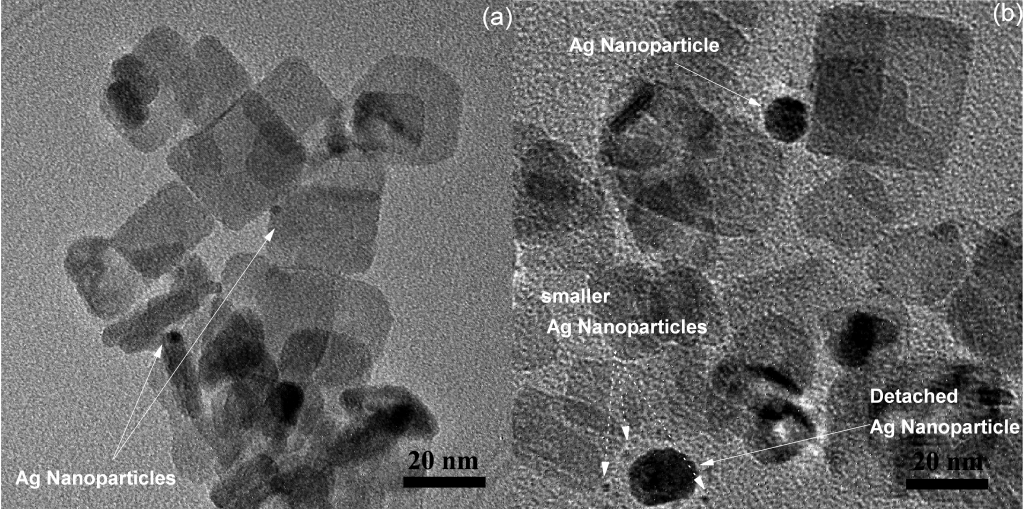
All Ag-decorated TiO2 nanoplates exhibited enhanced photocatalytic properties, regarding NO oxidation and acetaldehyde decomposition, in comparison to the pure TiO2 nanoplates due to the lower recombination rate of the photogenerated electrons and holes.
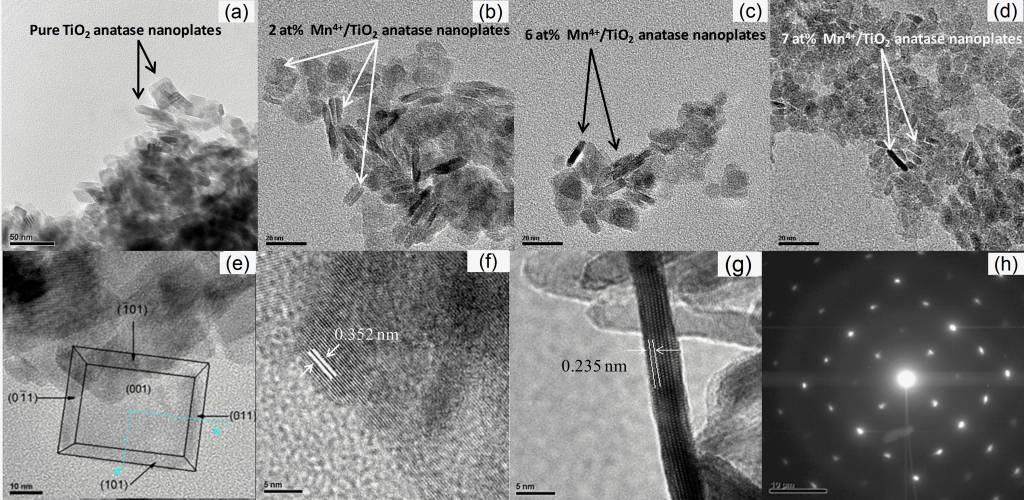
Morphosynthesis of magnesium and manganese ions doped anatase nanocrystals with exposed {001} facets The doped and undoped nanocrystals with exposed {001} crystal facets were synthesized by a solvothermal method. The morphological structure of TiO2 nanocrystals (a,b) changed with Mg2+ doping. The nanoplates became larger and pilled one on top of the other forming this way a separate bigger nanocrystal (c,d). As the content of Mg2+ ions increases, more nanoplates pile up making the crystal more dense and thicker (e,f). With the further increase of Mg2+ content in the anatase lattice the nanoplates are densely stocked together forming a sea-urchin morphological structure (g,h). The corresponding SAED patterns show how the nanoplates gradually lose their perfect alignment and perfect single crystal structure and become randomly oriented one on top of the other for the highest Mg2+ content.
The TEM micrographs of the doped nanocrystals with Mn2+ ions in different atomic ratios reveal that the obtained doped TiO2 product consists of uniform, well-defined plate-shaped structures possessing a rectangular outline. The HRTEM images (f,g) of the anatase samples viewed along the [001] and [010] crystallographic directions, as well as the SAED pattern (h) confirm that the square surfaces are {001} facets of the single-crystalline nanoplate (e).The photocatalytic tests that were performed to all samples revealed that Mg2+ and Mn2+ ion doping in small amounts causes better separation of electrons and holes thus enhancing the photocatalystic activity in comparison to the pure TiO2 nanoplates. Lastly, the manganese doped nanoplates showed photocatalytic activity towards the visible light region in comparison to the pure anatase sample.
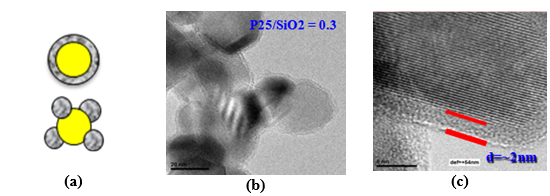
TiO2 surface modification: TiO2 core/inorganic SiO2 shell structuresCore-shell nanostructures with TiO2 core and aerogel SiO2 shell with controlled shell thickness and porosity were developed. Such surface modification aims protection and immobilization of TiO2 photocatalyst in different construction material substrates. Partial covering and porous shell structure allow “access” of the reactants and decomposition products to and from the TiO2 photocatalyst core.
TiO2 surface functionalization with organic compounds
Treatment with organic compounds such as oleic acid, oleylamine and organosilane endow titania nanonoparticles with specific properties like hydrophilicity or hydrophobicity, reduced mean size, improved dispersibility in polar and non-polar solvents, improved photocatalytic efficiency even under visible light irradiation. TiO2 nanoparticles modified with hydrophilic organosilane compound (3-(2-aminoethylamino)propyltrimethoxysilane) depict the best photocatalytic activity for NO oxidation and NOx removal.
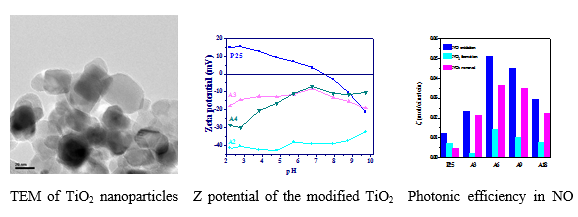
TiO2 nanoparticles grafted simultaneously with hydrophilic 3-(2-aminoethylamino) propyltrimethoxysilane and hydrophobic octadecyltriethoxysilane showed excellent photoactivity concerning De-NOx process in relation to bare photocatalyst. The incorporation of the TiO2 nanoparticles modified with the two mentioned modifiers in paints with low resin content, gave the best result in comparison with other modifiers as well as improved photocatalytic properties of the paint in comparison with the incorporation of bare photocatalyst. In order to obtain anisotropic and controllable hydrophobic properties biphase toluene/water emulsion processing was used. When amphiphilically modified nanoparticles with hydrophilic polyethylene glycol and hydrophobic oleylamine were incorporated in cement matrix, they depict excellent photocatalytic activity. The attached hydrophobic groups facilitate the accumulation of TiO2 at the cement surface and its exposure to irradiation and air pollutants, while the attached hydrophilic OH groups of polyethylene glycol enhance the De-NOx ability.
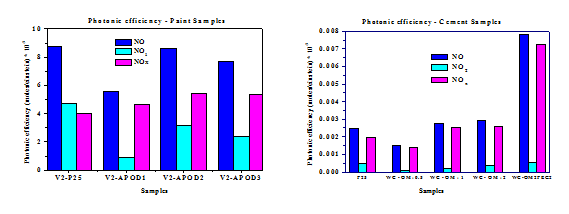
Photonic efficiency of photocatalytic cement with TiO2 modified with hydrophilic and hydrophobic modifiers in biphase solvent.
Nanomaterials by Flame Spray Pyrolysis
The flame spray pyrolysis (FSP) is a fast (one step) and versatile process for the production of a wide variety of oxide nanoparticles. A homemade FSP set-up was constructed and utilized to synthesize TiO2 pure and composite materials.Precursor solution is injected through the centre capillary of the FSP nozzle by a syringe pump at different rates. Oxygen is fed through the surrounding annulus as dispersion gas and a supporting CH4/O2 flame surrounding the oxygen gas annulus stabilizes the spray flame. A sintered metal plate ring provides an additional sheath flow (oxygen or nitrogen) surrounding the spray flame. The resulting powders are collected on glass microfiber filters with the aid of a vacuum pump. The relationship between the particle properties and the synthesis conditions (the combustion environment – excess or restrictive oxygen, the pump/precursor rate and the enthalpy combustion of the fuel) is studied.
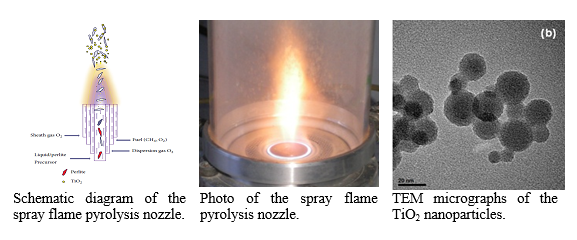
Although the surface specific area of the TiO2/perlite composites was lower than that of P25 sample, their photocatalytic activity in NO oxidation was comparable and even higher for some of them. This result was attributed to the presence of perlite glassy substrate that facilitates photocatalyst dispersion as well as to the synergistic effect between two crystalline phases.
Composite TiO2/ZnO films
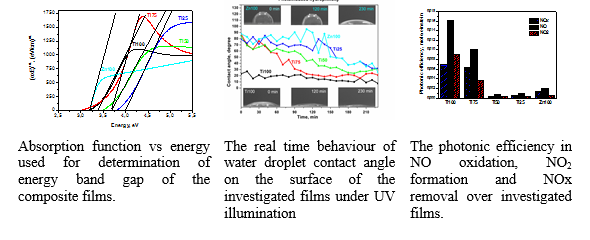
Composite TiO2/ZnO films were prepared targeting novel materials with improved photocatalytic and optical properties. The films were synthesized via sol-gel technique using Ti alkoxide and Zn acetate as metal precursors and dip coating deposition on quartz substrate.The optical characteristics were assessed via measurements of UV-vis spectra of the films and via modeling of their complex refractive index in the frames of popular dispersion models (Forouhi-Bloomer (FB) and Tauc-Lorentz (TL). The photocatalytic properties of the films were investigated by monitoring their photoinduced hydrophilicity and NOx oxidation activity under UV irradiation. Modelling of the films’ optical constant via FB and TL models allowed determination of their energy band gap and thickness. The recorded blue shift of the absorption edge of composite films Ti25 and Ti50 was attributed to Burstein-Moss effect concerning heavy doped semiconductors. Under UV illumination, the hydrophilicity of all the films gradually increased with the change for the ZnO containing films to be more prominent. The pure TiO2 film exhibited the highest photocatalytic activity especially in NO to NO2 oxidation.
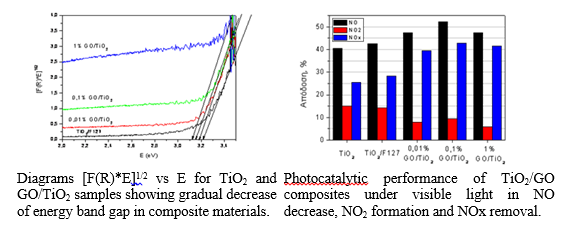
Graphene/TiO2 composite materials
Exfoliated graphene and graphene oxide were separately introduced in TiO2 matrix in order to increase the photocatalytic efficiency of the photocatalyst through enhanced charge carriers separation. The structural properties and the photocatalytic activities of TiO2/G and TiO2/GO composites in various ratios were investigated. The TiO2/GO materials exhibited significant improvement in the NOx removal from ambient air especially under visible light irradiation. Coupling of graphene with TiO2 in the form of anatase with highly active {001} crystallographic facets was achieved via hydrothermal processing.
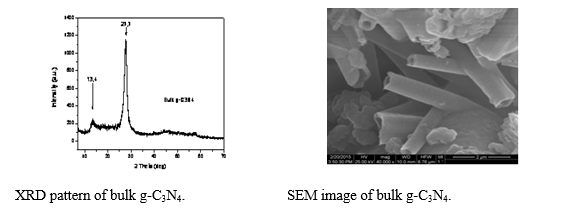
One-step fabrication of graphitic carbon nitride (g-C3N4) hybrids
Graphitic carbon nitride was prepared by direct pyrolysis of melamine under Ar atmosphere. Various temperatures and heating durations were tested, until the optimization of the final product was achieved. The obtained material appears to be really hard and revealed an interesting tube-like morphology.
Melamine was mixed with TiO2 in various ratios and pyrolyzed in order to receive g-C3N4/TiO2 hybrids. These hybrids showed a really good and stable photocatalytic activity which opens a whole new range of possibilities in the field of air pollutants removal.
Chemical and thermal exfoliation of g-C3N4 for efficient NOx removal
Melamine-derived g-C3N4 was exfoliated by high-yield chemical and thermal treatment. A thorough comparative investigation was performed, revealing successful exfoliation of g-C3N4 and significantly enhanced visible light photocatalytic activity. Both methods led to high pore volume and specific surface area. It was established that chemical exfoliation resulted in wider band gap with more positive VB edge in comparison to thermal exfoliation. Furthermore, increased superoxide radical formation and reactivity of photogenerated electrons was demonstrated by EPR measurements in the case of chemical exfoliation.Regarding NOx removal, the chemically exfoliated g-C3N4 showed superior photocatalytic activity under visible light irradiation. This was attributed to the favorable band gap edges, the enhanced formation of superoxide radicals and accelerated transfer of pollutants due to the larger pore volume and specific surface area. Comparing the two exfoliation methods, thermal treatment offers a facile and inexpensive way of improving the photocatalytic activity of g-C3N4, whereas chemical treatment demands multiple steps but leads to excellent NO oxidation and superior NOx removal.
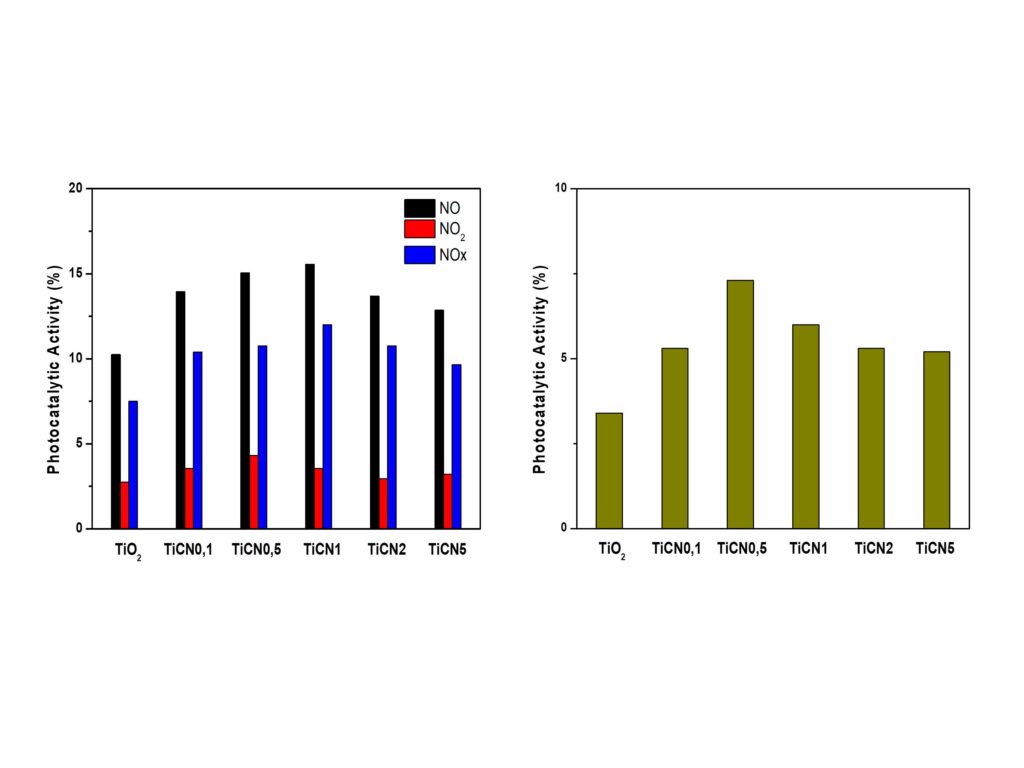
Novel photocatalytic coatings for air pollutants oxidation and antibacterial applications
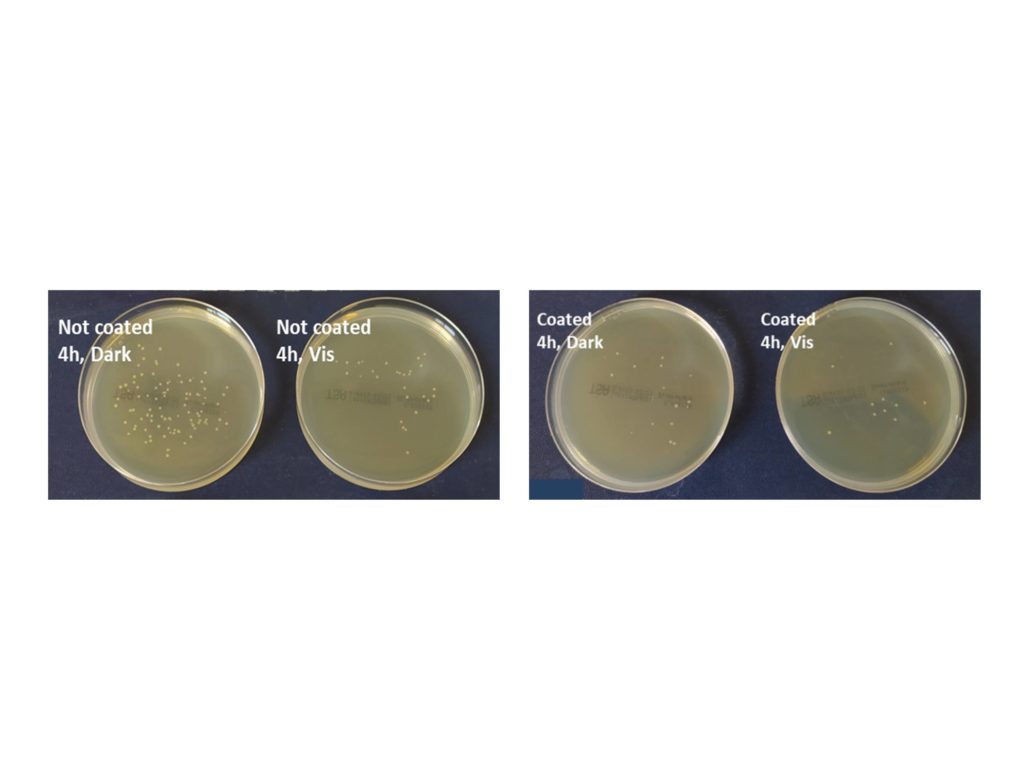
Novel photocatalytic coatings were prepared in collaboration with NanoPhos SA, by adding small amounts of g-C3N4 into a known commercial product. The resulting suspensions were deposited on a-block substrates of suitable dimensions using the Z-020-Tornador BLACK sprayer and then studied for air pollutants oxidation and antibacterial activity. The addition of g-C3N4 significantly enhances the photocatalytic activity of the coatings. Particularly under visible light irradiation, the TiCN0.5 and TiCN1 coatings show twice the activity of the commercial TiO2 coating, both for NOx removal and acetaldehyde oxidation. As it stands, addition of just 0.5 wt% of g-C3N4 is sufficient for the improvement of the photocatalytic activity.
As can be seen, after irradiation the coated samples show almost three times fewer colonies than the non-coated samples. This activity is attributed to the photogenerated ·O2– and ·ΟΗ radicals that are formed on the surface of the photocatalysts. These radicals are responsible for the oxidative "stress" against the microorganisms, thus leading to the reduction of the grown colonies.
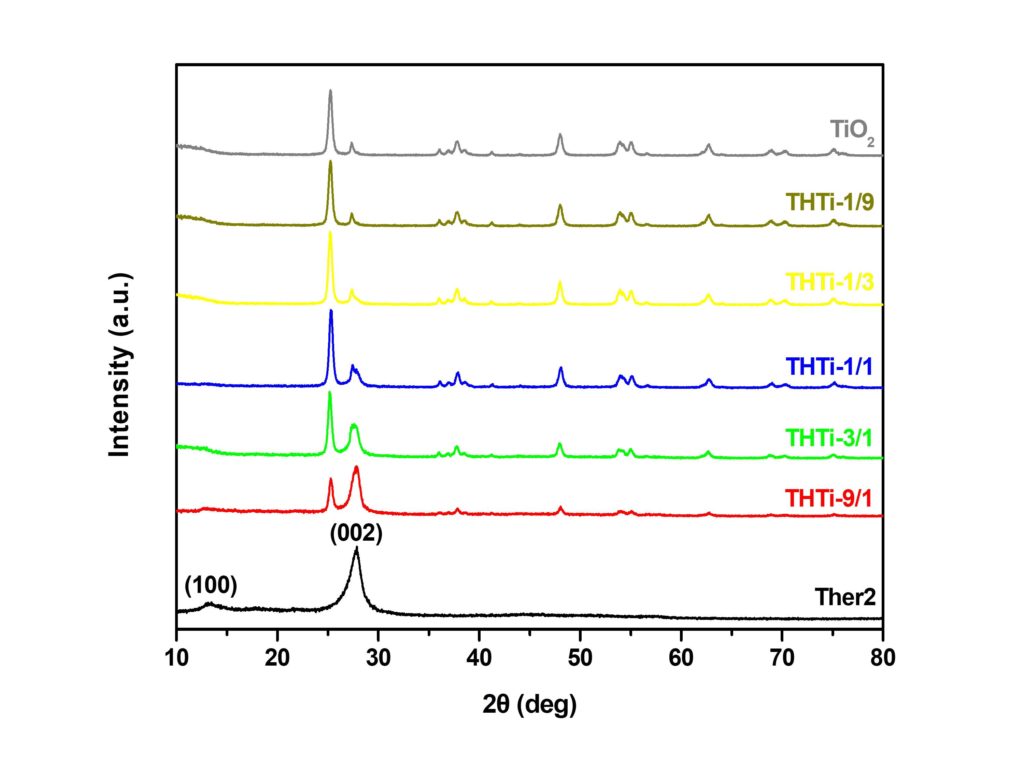
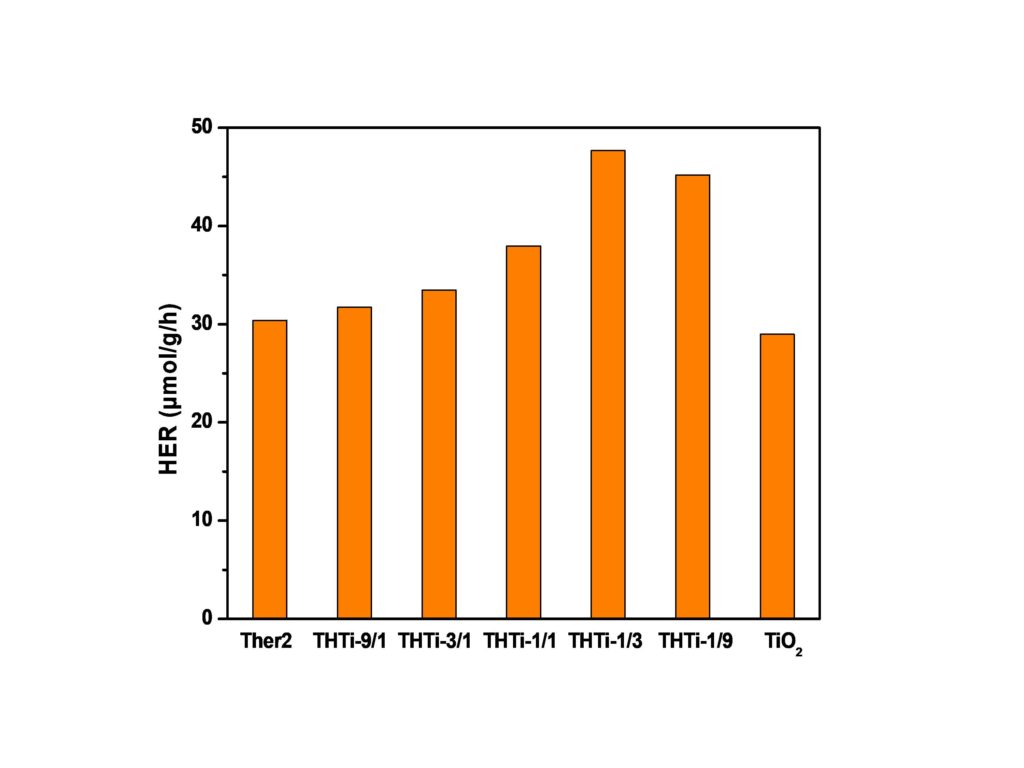
Development of g-C3N4/TiO2 heterostructures for H2 production
Novel g-C3N4/TiO2 heterostructures were prepared by mixing thermally exfoliated g-C3N4 and commercial TiO2 in different ratios. The XRD and FT-IR results confirmed the presence of both photocatalysts. The effective band gap energy was also changed depending on the g-C3N4 : TiO2 ratio. The heterostructures were examined for photocatalytic hydrogen production under UV and visible light irradiation utilizing a home-made glass reactor.The heterostructures with higher g-C3N4 concentration showed the best photocatalytic activity under visible light irradiation, achieving HER of 50 μmol/g/h. The improved behavior of the photocatalysts originated from the combined effect of increased specific surface area (SSA) and lack of surface defects. The increased SSA provides additional active sites while the elimination of defects prevents the recombination of photogenerated charge carriers.