Single Crystal Crystallography
The laboratory is a well-recognized crystallography laboratory worldwide as it is proved by the more than 1400 cif files deposited in the data base of the Cambridge Crystallographic Data Centre (CCDC) since its establishment in the late seventies. During nineties the laboratory had played a national role through the collaborations that have been developed with all the Inorganic Chemistry departments of Greek Universities. On the occasion of retirement of Dr. Aris Terzis, the ex-director of the laboratory, a special issue was hosted by the Polyhedron journal with the title: The Impact of Crystallography on Inorganic Chemistry in Greece ([Polyhedron, Volume 28/issue 15 2009, doi:10.1016/j.poly.2009.09.013].

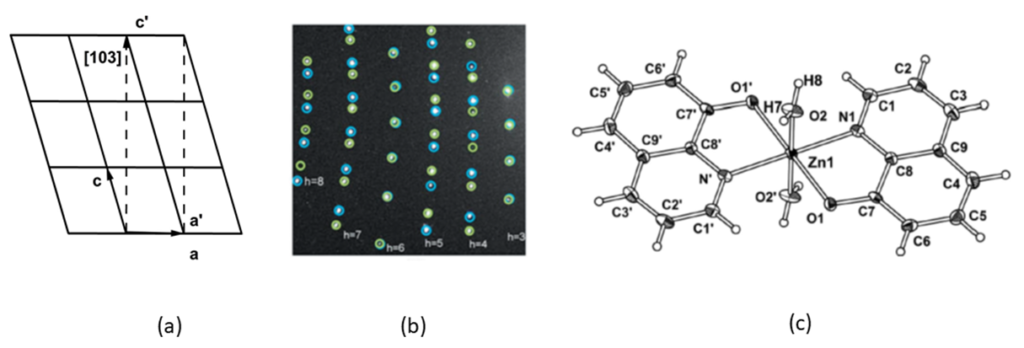
During all these years the personnel has developed its skills by using all the available software and methods for structure studies and analysis. The structures studied and analyzed are disordered or are obtained from twined crystals. The details of the packing of the structures are studied by using traditional tools and also by using modern one such as Hirshfeld surface analysis tools. In Figure-1 the direct, the reciprocal lattice relationships and the final structure model from a non-merohedral twin crystal is presented as it is presented in the paper [Acta Crystallographica, C69, pp. 868-871, 2013, doi: 10.1107/S0108270113018386].were the methodology to treat data from nomerohedral twin crystal is presented.
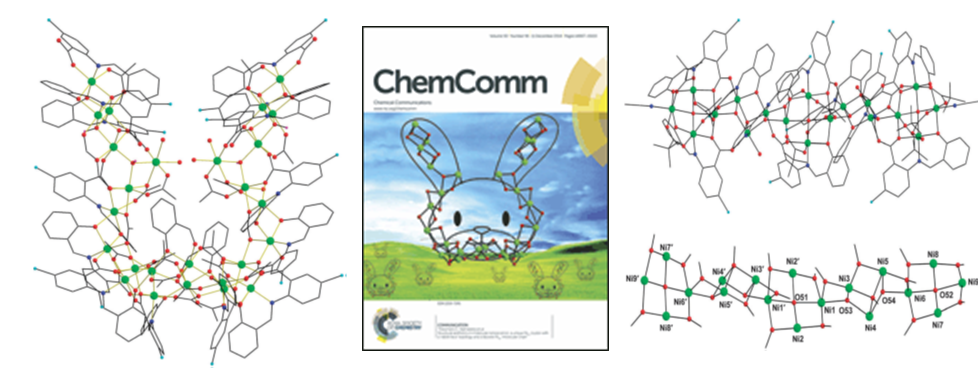
During the last years many structures with interesting topologies have been studied and published in prestigious journals. The crystallogrpahic characterization of a unique Ni26 cluster with a 'rabbit-face' topology (Figure 2) and a discrete Ni18 'molecular chain' (Figure 2) was reported in collaboration with Brock University, Canada and University of Barcelona, Spain [Chem. Commun. (2014) 50, 14942-14945, doi: 10.1039/C4CC07192A]. The Ni26 cluster adopts a saddle-shaped conformation with a diameter of ~24 Å. The Ni18 'molecular chain' has a nanotubular structure with a length of ~35 Å and a thickness of ~16 Å. The work was chosen for the front cover of the 11th December 2014 issue of Chemical Communications.
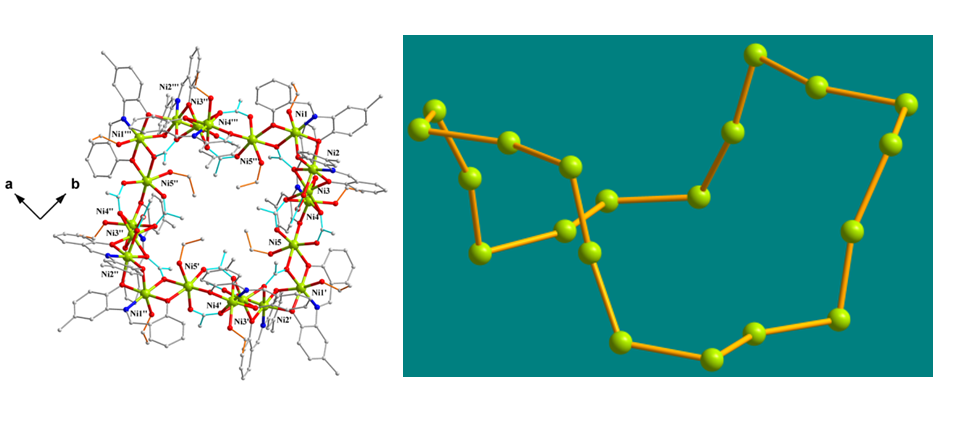
The crystallographic characterization of a NiII20 cluster with an unprecedented 'bowl' metal topology was reported in collaboration with the University of Patras, Greece and the University of Barcelona, Spain [Inorg.Chem. 54 (2015) 5615-5617), doi:10.1021/acs.inorgchem.5b00521].The saddle-shaped molecule is disposed around a crystallographically S4 axis. Magnetic susceptibility studies revealed a S=12 ground state as a result of combined ferro- and antiferromagnetic interactions.
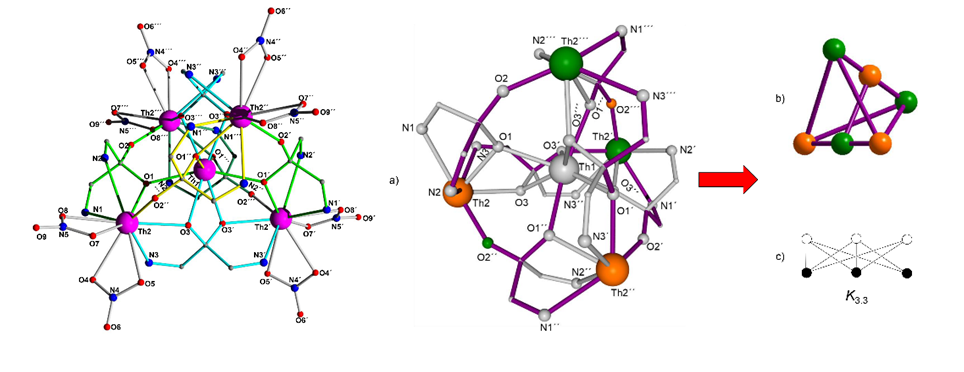
Recently, the first Pentanuclear Thorium(IV) coordination cluster presenting a Kuratowski type topology was studied in collaboration with the University of Patras [Inorganic Chemistry 2021, 60, 16, 11888-11892, doi: 10.1021/acs.inorgchem.1c01800]. . This topology creates holes in a structure and it is useful in the synthesis of MOFs.
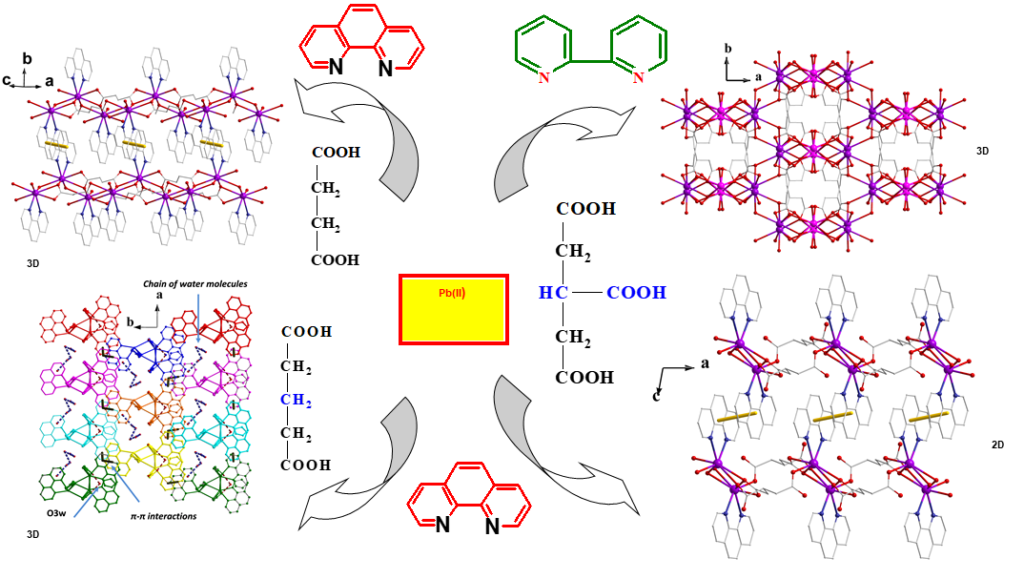
The crystallographic characterization of materials reveals also the packing characteristics of the studied compounds and this information is valuable in the study of metal-organic frameworks (MOFs). A series of MOFs was studied in collaboration with the Aristotle University of Thessaloniki, Greece, the Leipzig University, Germany and the Banat's University, Romania [Cryst.Growth&Des 15 (2015) 1666-1682 DOI: 10.1021/cg501628d]). Pb(II)-(di)-tricarboxylate ligand systems in the presence of variable-nature aromatic N,N'-chelators gave 2D and 3D frameworks of distinct architecture and dimensionality. The crystal structure of these MOFs is built due to hydrogen bonding and π-π interactions and is thoroughly discussed. The photoluminescent properties of the MOFs showed that these are probably affected by the nature of the (di)-tricarboxylic acid ligand for the same or similar structural components in the repeating units of the polymeric materials. Considering that none of the free ligands exhibit any luminescence at r.t., these compounds can be regarded as potential luminescent materials.
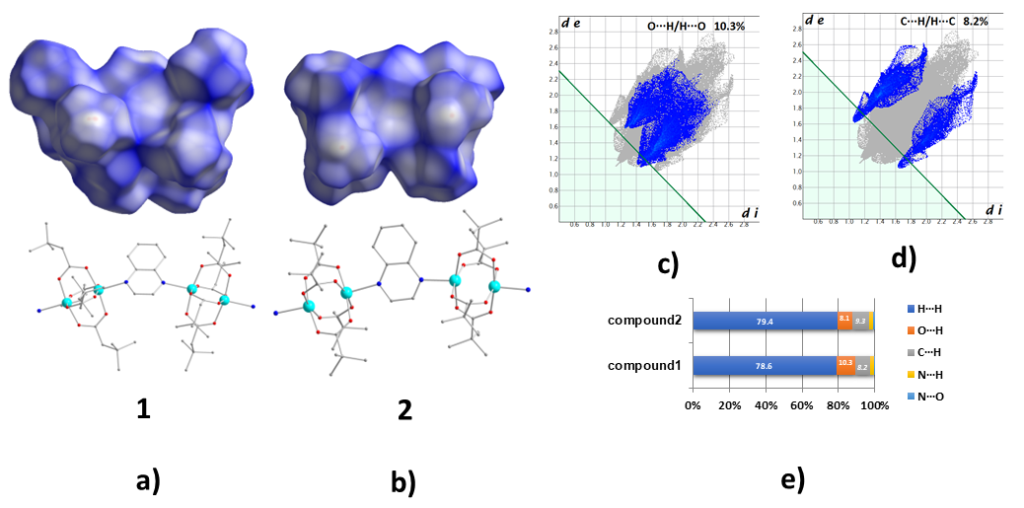
As the packing characteristics of a structure are important for understanding the structure peculiarities and properties the Hirshfeld surface analysis tools was used in the study of 1D polymeric complexes {[Cu2(diba)4(qunx)]}n and {[Cu2(piv)4(qunx)]}n a work performed in collaboration with Chemistry Department of Patras University Dalton Trans. (2017) 46, 260-274, doi:10.1039/C6DT03595G].The structure study of both compounds have revealed the new chemical transformation of acacdoH2 → qunx and a mechanism has been proposed based on well-established reactions of organic chemistry. Both complexes have similar molecular structures consisting of paddle-wheel {Cu2(η1:η1:μ-O2CR)4} units bridged by qunx ligands in a zigzag 1D chain arrangement. Hirshfeld surface analysis reveals the importance of C-H···O intrachain intermolecular interactions which characterize both the structure and the magnetic properties of both compounds and also the importance of C-H···π interactions in layer formation by the chains. Dc magnetic susceptibility studies in the 1.8-310 K range reveal very strong antiferromagnetic CuII…CuII exchange interactions within the carboxylate-bridged Cu2 units and weaker antiferromagnetic interactions between the Cu2 units via the qunx superexchange pathways.
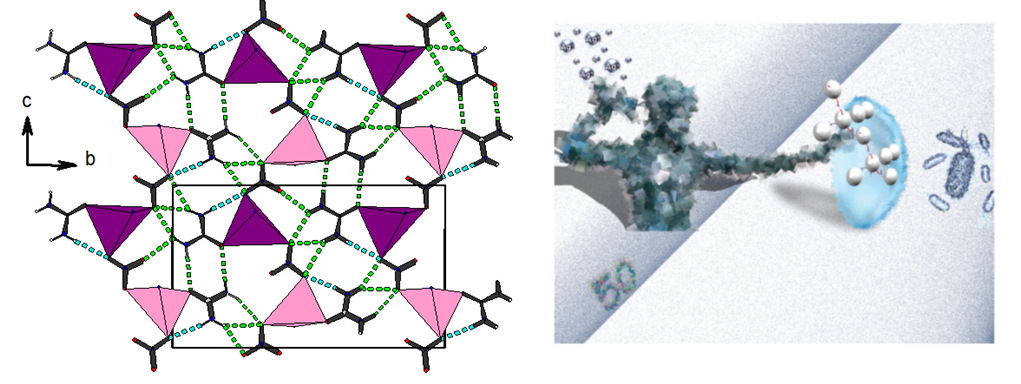
In a recent collaboration with the group of Prof. Hadjikakou (Laboratory of Biological Inorganic Chemistry, Department of Chemistry, University of Ioannina, we have studied the structure of Silver-Nitrate Urea compound Figure-7, which together with a series of silver(I) covalent polymers were tested for their antibacterial efficiency against microbes which colonize in contact lenses [Dalton Trans 50 (2021) 13712-13727, 10.1039/D1DT02158C ]. The specific compound presents moderate activity but the others present superior one. The graphical abstract of this work was selected as the back cover of the Issue 39, 2021 of Dalton Transactions journal.