Synthesis of Molecular Magnetic Materials
Polynuclear coordination clusters and coordination polymers containing paramagnetic metal ions may exhibit magnetic characteristics which makes them suitable for potential applications such as high storage magnetic recording media, quantum computing, spintronics and magnetic refrigeration. The goals of our synthetic work towards molecular magnetic materials are to explore the coordination chemistry of the chosen paramagnetic metals and ligands and use the knowledge gained in order to modify our synthetic approaches to prepare new materials with the desired magnetic behavior (e.g. ferromagnetic exchange coupling, large spin in the ground state, single molecule magnetism, single chain magnetism, etc). Our activity has resulted in the synthesis of Single Molecule Magnets, Single Chain Magnets, Molecular Magnetic Refrigerants and Chiral Molecular Magnetic Materials.
This activity is performed in collaboration with the groups of Superconducting and Magnetic Oxides, and Molecular Magnetic and Bioinorganic Spectroscopy at INN. The impact of this activity at international level is confirmed by two international workshops, North America-Greece-Cyprus Workshop on Paramagnetic Materials and Current Trends in Molecular and Nanoscale Magnetism, which are organized every two years since 2005, and also by two book chapters concerning ac magnetic susceptibility measurements and other spectroscopic techniques in Single Molecule Magnets: Molecular Architectures and Building Blocks for Spintronics, Wiley-VCH, 2019. (ISBN:9783527809929, https://onlinelibrary.wiley.com/doi/book/10.1002/9783527809929 ).
Single Molecule Magnets (SMMs)
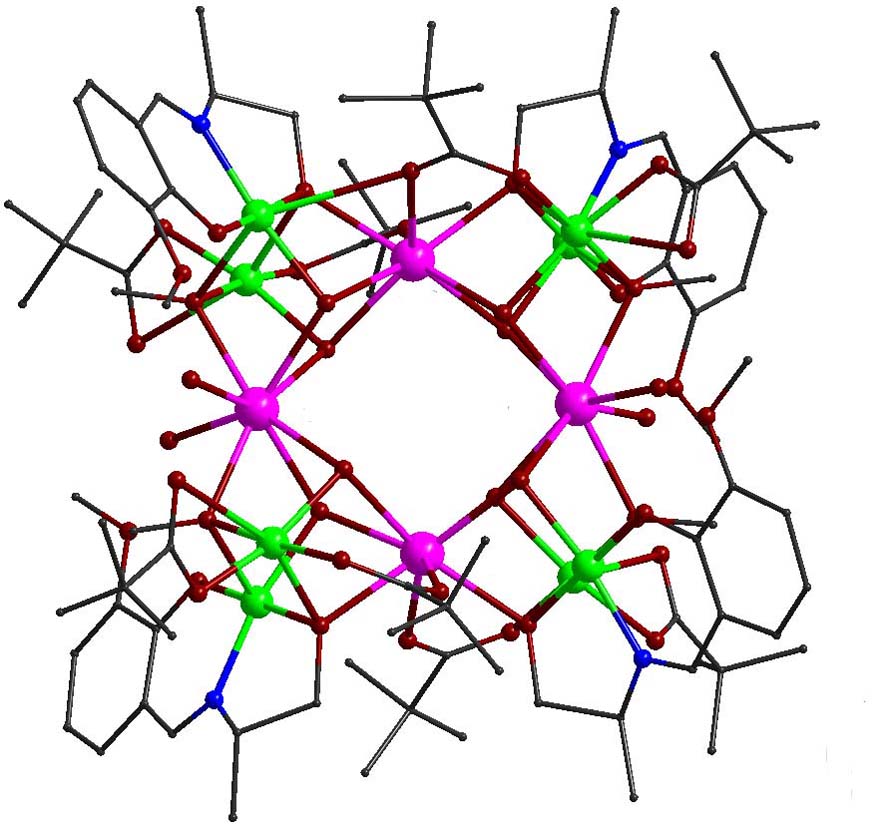
Polynuclear clusters that exhibit single molecule magnet behavior, as a result of the combination of large spin in the ground state and large Ising-type magnetic anisotropy (negative zero field splitting parameter D), may display significant energy barrier for reorientation of the magnetization vector, U = S2|D| and (S2-1/4)|D| for integer and half-integer total spin, respectively. This means that the molecule could be trapped in one of the two lowest energy states and ‘remembers’ the sense of the field that was applied to it.The combination of 3d/4f metal ions in one molecule can achieve high spin ground states (via the involvement of the 3d metal ions) along with large single-ion anisotropy (via the presence of the 4f metal ions) and provides, in many cases considerable energy barrier for magnetization reversal leading to SMM behavior. Within this respect, heterometallic complexes of the general formula [Cu5Ln2(O2CMe)2(NO3)4(L)2(HL)2] (Ln(III) = Tb, Dy, and H3L are Schiff base ligands) were prepared. The complexes consist of two distorted centrosymmetrically related cubane subunits, {Cu3Ln(μ3-OR)3(μ3-O2CMe)}5+, with common apex a Cu(II) ion. Peripheral ligation and bridging are provided by the Schiff base ligands, the acetato and nitrato anions. AC susceptibility measurements revealed slow relaxation processes suggesting the presence of SMM behavior [Polyhedron, 2018, 150, 47-53, doi: 10.1016/j.poly.2018.05.003; Polyhedron, 2019, 169, 135-143, doi: 10.1016/j.poly.2019.05.004].
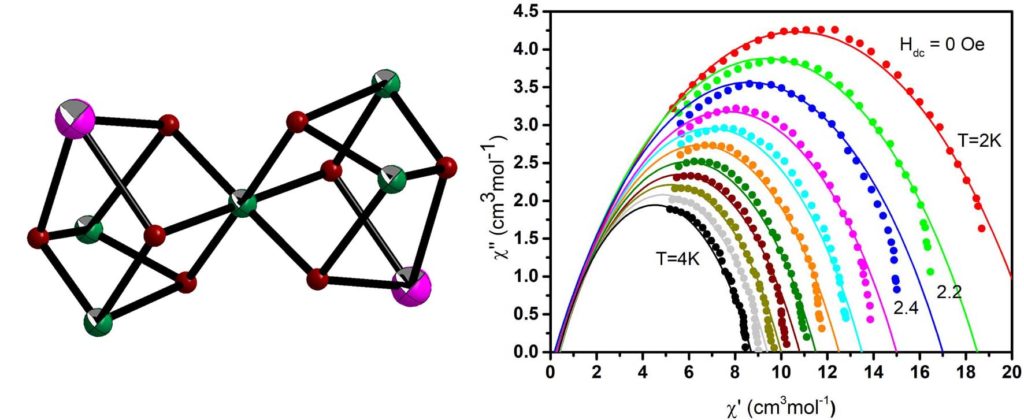
The linear trinuclear complex [Ni2Dy(H3L)4(O2CMe)2](NO3) (H4L is Schiff base ligand) crystallize in the non-centrosymmetric space group Pc21n and consists of three metal ions in almost linear arrangement. Under HDC = 1.0 kOe the complex displays SMM behavior. The relaxation properties of the complex are governed by the direct and Orbach processes with parameters A = 13.0(2)×103 K-1s-1 and U = 26(2) K (τ0 = 4(2)×10-8 s), respectively [Crystals, 2022, 12, 95, doi:. 10.3390/cryst12010095].
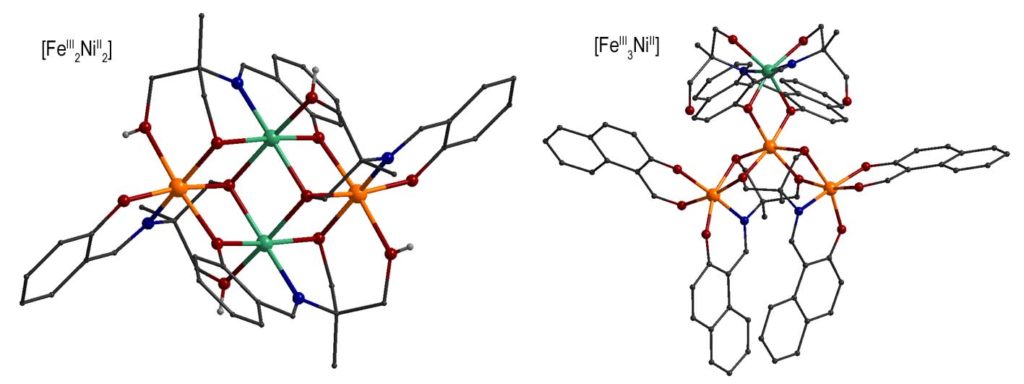
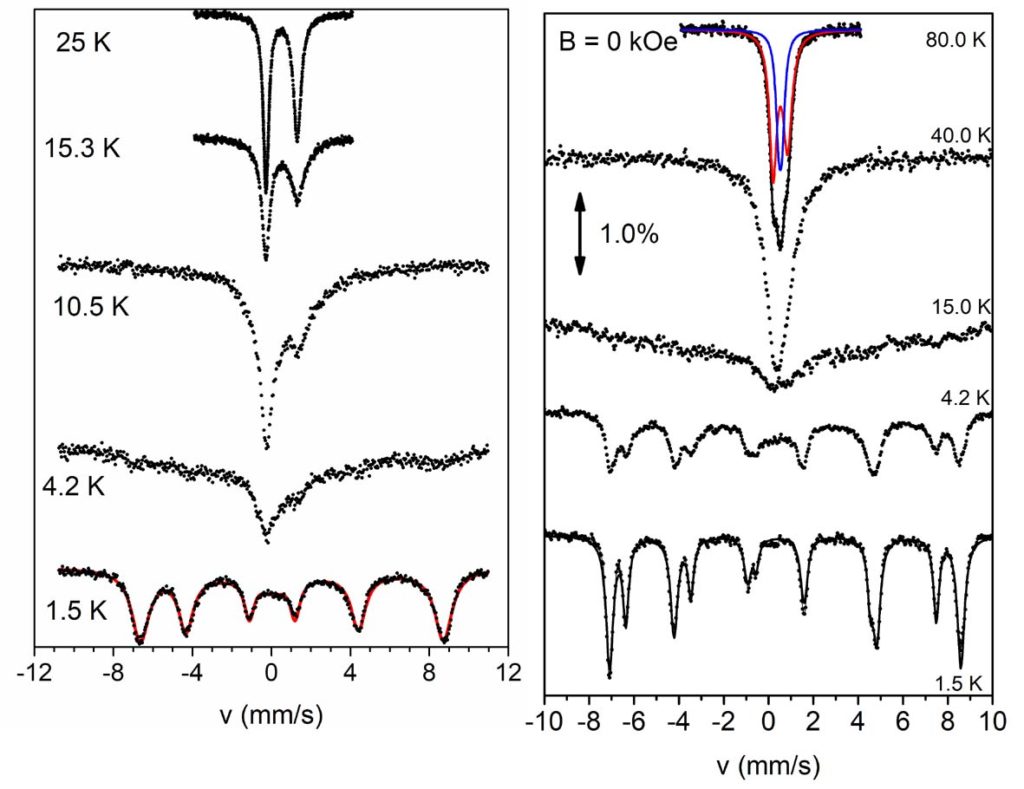
By using Schiff base ligands and following a different synthetic approach, heterometallic 3d/3d’ complexes have been prepared, i.e. {FeIII2NiII2} complexes with ‘butterfly’ metal topology and {FeIII3NiII} complexes with propeller structure or ‘star’ which exhibit ground state spin S = 3 and S = 7/2, respectively. The dynamic magnetic properties of the complexes revealed that the imaginary part of the magnetic susceptibility deviates from zero, under external magnetic field. This behavior is indicative of the presence of slow relaxation of the magnetization below 2 K, with Ueff/kB ~ 6-7 K (τ0 ~ 10-8 – 10-7 s). These complexes were examined in detail by Mössbauer spectroscopy in the temperature range 1.5-300 K. The complexes showed magnetic spectra comprising of characteristic sextets below 4.2 K which suggests that they behave as SMMs in the Mössbauer time scale.
Single Chain Magnets (SCMs)
One-dimensional coordination polymers which exhibit slow relaxation of the magnetization below a critical temperature, and thus magnetic bistability, have been termed in the literature as Single Chain Magnets (SCMs). These chain-like molecules do not interact magnetically to each other, exhibit strong intra-chain exchange interactions and display 1D Ising ferro- or ferri-magnetism.In order to prepare new 1D materials with SCM behavior a synthetic approach that enables polymerization of preformed SMMs was followed. The 1D coordination polymer [MnIII6O2(ter)(salox)6(dmf)4]n.2ndmf was prepared by replacing the benzoato ligands in the SMM [MnIII6O2(O2CPh)2(salox)6(Me2CO)2(H2O)2] with terephthalato dianions. The magnetic study of the 1D polymer revealed that it behaves as SMM and no evidence of SCM behavior was observed [Polyhedron, 2013, 52, 917-923, doi:10.1016/j.poly.2012.07.022].
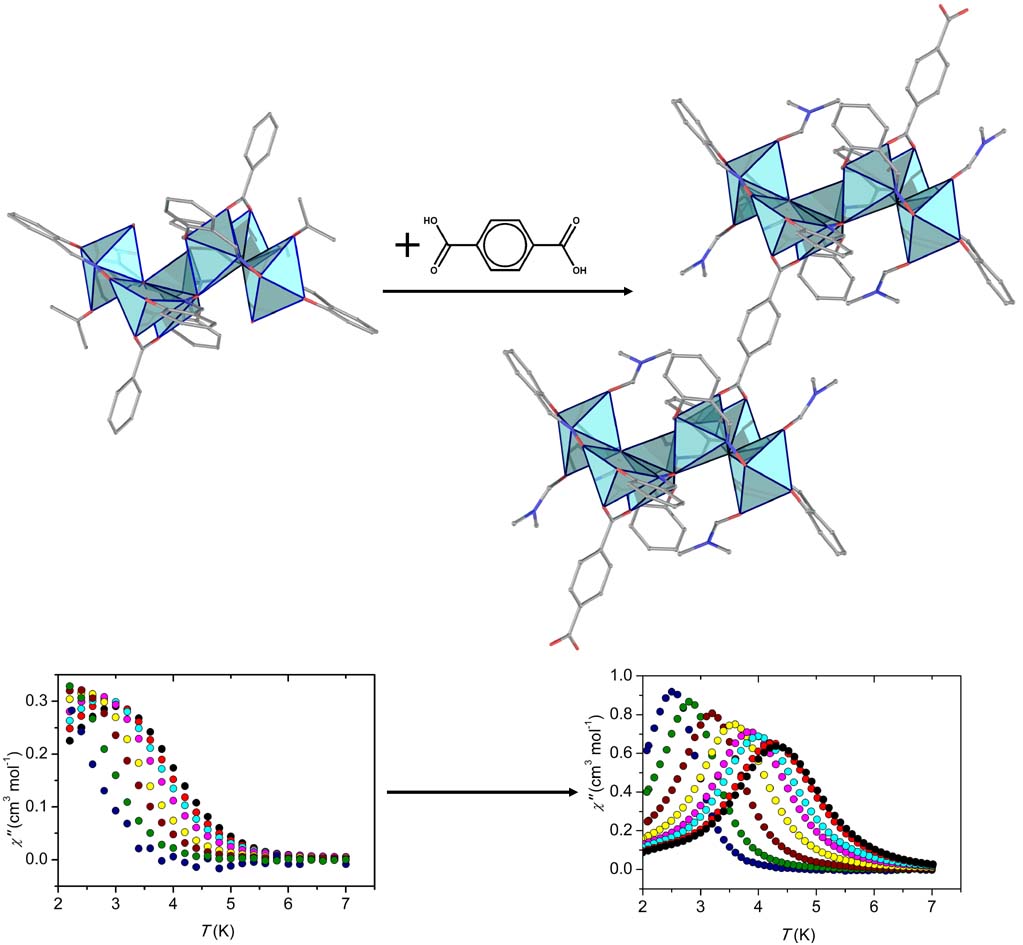
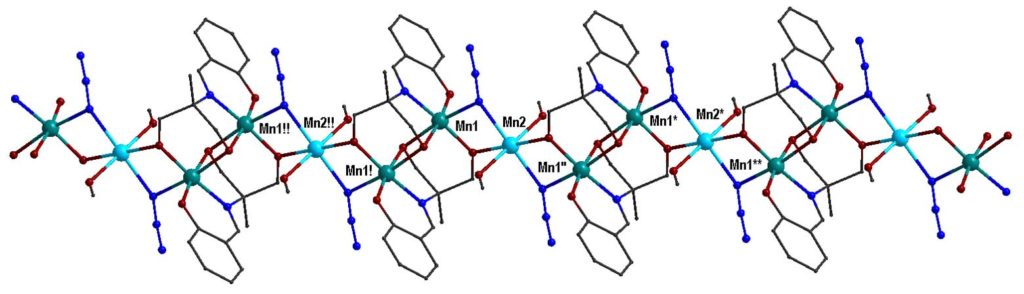
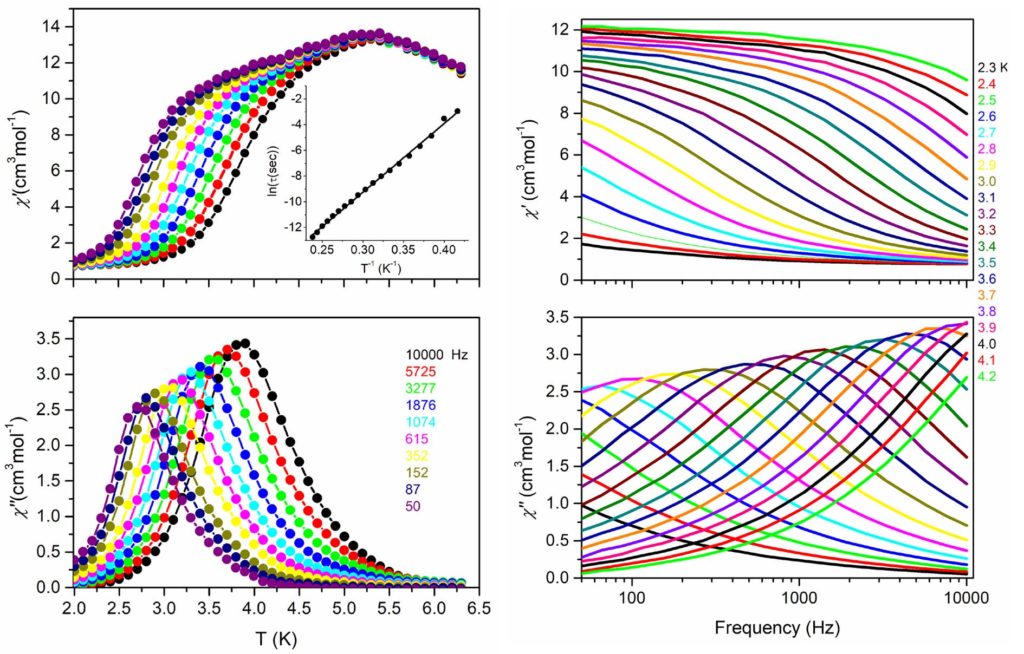
By using the synthetic approach of ‘serendipitous assembly’ a 1D polymer, [MnIII2MnII(L)2(N3)2(MeOH)2]n, (H3L is a Schiff base ligand) was prepared, which consists of Mn(III)-Mn(II)-Mn(III) strictly linear trinuclear repeating units with bis(end-on azido/alkoxo) bridges between the central Mn(II) and each one of the terminal Mn(III) ions. The magnetic studies revealed that the 1D polymer displays SCM behavior with energy barrier Δ = 53±1 K. This compound is the first example of SCM based on a mixed-valence linear trinuclear unit [Polyhedron, 2021, 206, 115334, doi: 10.1016/j.poly.2021.115334].
Molecular Magnetic Refrigerants
Molecular Magnetic Materials that exhibit large Magnetocaloric Effect (MCE) have been proposed as materials for achieving magnetic cooling at low temperatures as an alternative process of using liquid He, mainly due to the depletion of helium reserves and its high cost. The magnetic cooling is based on the magnetocaloric effect, i.e. the isothermal change of magnetic entropy (ΔSm) and adiabatic change of temperature (ΔTad) that follow a change of the applied magnetic field (ΔH). Materials with high MCE achieve greater temperature difference, hence the material is suitable for magnetic cooling applications. Magnetic refrigeration requires molecules possessing a large spin ground state and negligible magnetic anisotropy. We have prepared heterometallic Cu/Ln and Ni/Gd complexes as potential molecular magnetic refrigerants.Heterometallic complexes of the general formula [Cu5Gd2(O2CMe)2(NO3)4(L)2(HL)2] (H3L are Schiff base ligands) consist of two distorted centrosymmetrically related cubane subunits, {Cu3Ln(μ3-OR)3(μ3-O2CMe)}5+, with common apex a Cu(II) ion. Peripheral ligation and bridging are provided by the Schiff base ligands, the acetato and nitrato anions. The magnetocaloric effect was examined via isothermal magnetization measurements under various applied fields and the maximum entropy change was found in the range 15.7-16.3 Jkg-1K-1 at 2 K for ΔH = 5 T [Polyhedron, 2018, 150, 47-53, doi: 10.1016/j.poly.2018.05.003; Polyhedron, 2019, 169, 135-143, doi: 10.1016/j.poly.2019.05.004].
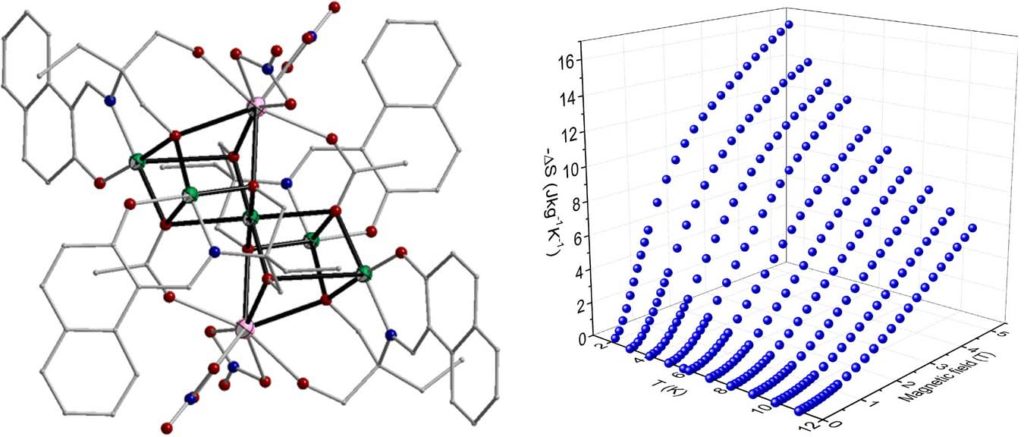
Heterometallic complexes of the general formula [Cu7Gd2(piv)4(L)4(HL)2(solv)4/5] (H3L are Schiff base ligands) have been also examined as potential molecular magnetic refrigerants. Isothermal magnetization measurements under various applied fields revealed that the maximum entropy change is found in the range 12.1-13.2 Jkg-1K-1 at 2 K and ΔH = 5 T [Polyhedron, 2021, 195, 114960, doi: 10.1016/j.poly.2020.114960].
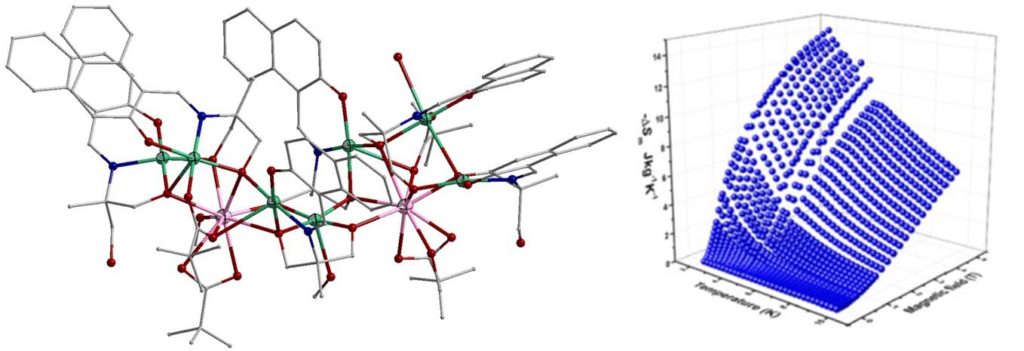
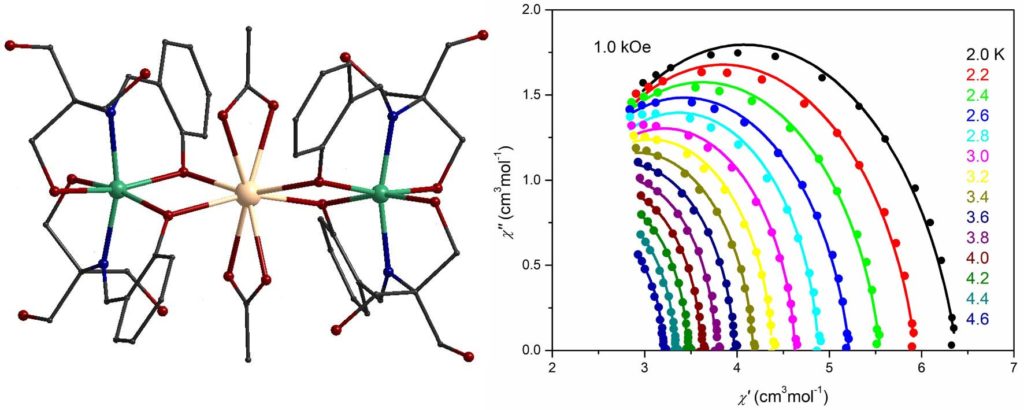
A linear trinuclear complex [Ni2Gd(H3L)4(O2CMe)2](NO3) (H4L is Schiff base ligand) showed maximum magnetic entropy change of 14.2 Jkg-1K-1 at 2 K [Molecules, 2020, 25, 2280, doi:10.3390/molecules25102280].
Chiral Molecular Magnetic Materials
Modern technological requirements in the field of nanotechnology require the synthesis of multifunctional materials which combine different properties. Materials that behave as Single Molecule Magnets (SMMs) and simultaneously exhibit ferroelectric/dielectric behavior and chirality have been developed in recent years. A basic condition for the appearance of ferroelectric behavior from a crystalline material is to crystallize in one of the 10 polar point groups, and this can be achieved by synthesizing enantiomeric complexes. The chances of synthesizing such complexes are increased if only one of the two enantiomers of the ligands are used in the synthetic reactions.By using chiral Schiff base ligands, enantiomerically pure families of {Cu8Ln4} complexes have been successfully prepared, whose structures consist of four corner sharing {Cu3Ln} cubane subunits.