Synthetic Coordination Chemistry
Our research is focused on the synthesis and characterization of polynuclear coordination clusters and coordination polymers of paramagnetic 3d transition metal ions and 4f lanthanide ions. The coordination and bridging of the metal ions is provided by polydentate ligands, such as various Schiff bases, pyridyl-oximes and pyridyl-ketones, which contain N,O-donor atoms for chelation around the metal ions and also donor groups, suitable for metal bridging, thus increasing the nuclearity of the compounds.
By varying the synthetic parameters of the reactions, we are able to modify the identity of the compounds, the nuclearity of the metal clusters and the dimensionality of the polymers or supramolecular networks. We have prepared homometallic 3d metal clusters, and heterometallic 3d/3d’ and 3d/4f metal clusters with metal topologies ranging from molecular triangles, squares, hexagons and larger ring structures, cubanes and defective dicubanes, corner sharing cubanes and square pyramids, propeller structures and more complicated ones.
Coordination compounds based on Schiff base ligands
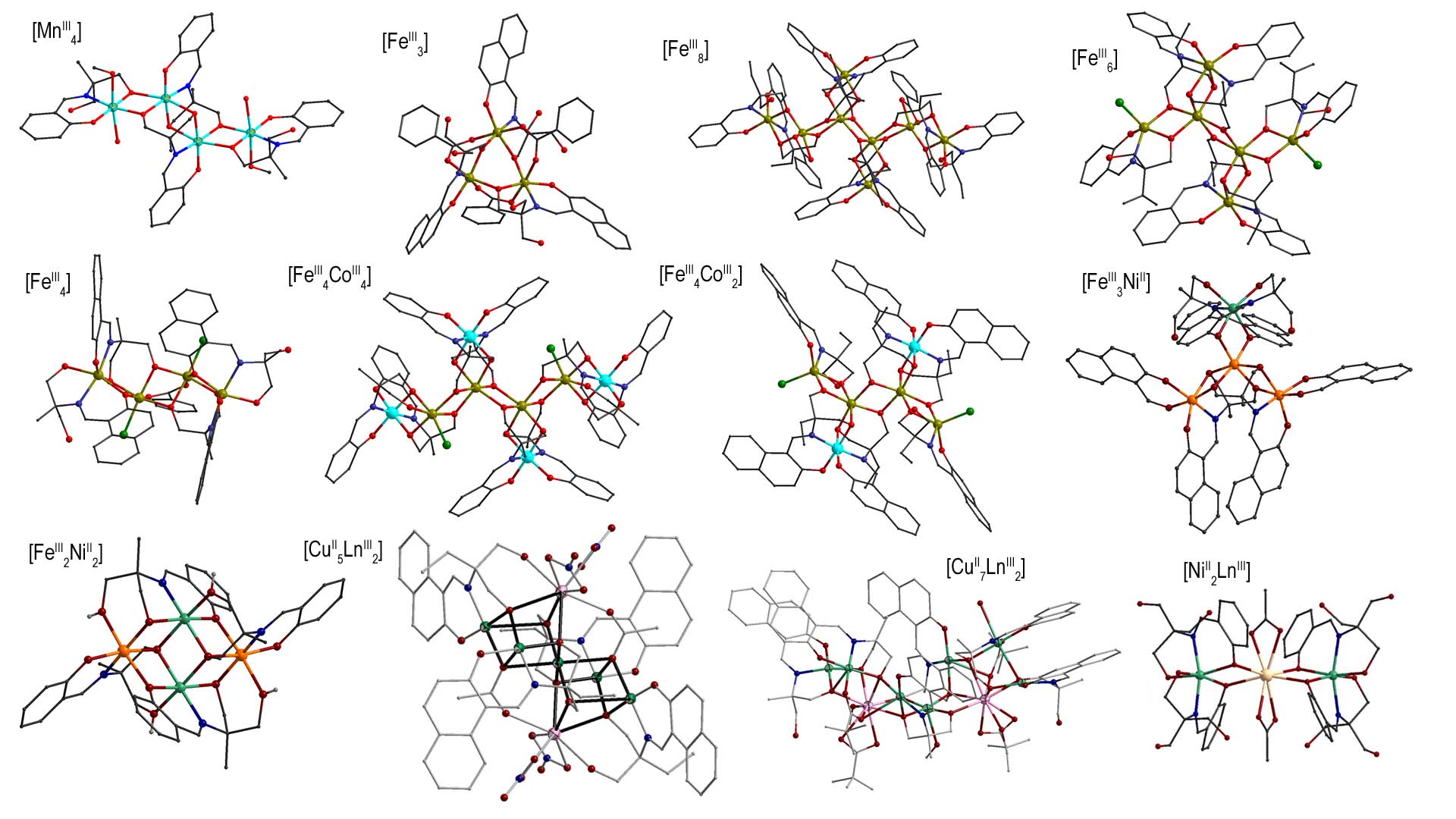
Our major synthetic work is focused on the use of various Schiff base ligands which are the condensation products between aldehydes (e.g. salicylaldehyde, 2-hydroxy-naphthaldehyde, o-vanillin) and amino-polyalcohols. These ligands contain a pocket of N,O-donor atoms, which can chelate around the metal ions, and also bear pendant alkoxide groups, which can be used as bridging groups between the metal ions. The pendant alkoxides offer various degrees of deprotonation, thus in the partially deprotonated forms, they participate in extensive hydrogen bonding interactions and form supramolecular networks of complicated topologies.By using Schiff base ligands, families of tetranuclear Mn(III) complexes have been isolated, in which the metal ions are bridged via deprotonated alkoxo-O atoms and form rhombic subunits [Mn2(OR)2] with zig-zag topology due to the presence of a center of symmetry in the central rhombic subunit. The evaluation of the magnetic behavior of these complexes was based on the results of magnetostructural correlations, according to which the magnetic coupling in dinuclear Mn(III) complexes with alkoxo bridges ranges from ferro- to antiferro-magnetic depending on the relevant arrangement of the Jahn-Teller axis [Polyhedron, 2013, 64, 181-188, doi: 10.1016/j.poly.2013.03.055]. Tetranuclear cubane and dinuclear copper(II) complexes have been prepared, which form interesting supermolecular lattices due to the extensive network of H-bonds [Polyhedron, 2018, 152, 125-137, doi: 10.1016/j.poly.2018.06.001]. Iron(III) complexes with various Schiff base ligands have been synthesized and isolated triangular complexes, families of polynuclear clusters with nuclearities from 3 to 8 and structural core consisting of rhombic subunits [Fe2(OR)2] [Curr.Inorg.Chem., 2017, 7, 66-85, doi: https://doi.org/10.2174/1877944107666170914113838], heterometallic iron(III)/cobalt(III) complexes with metal cores analogous to those of the respective homometallic complexes, as well as iron(III)/nickel(II) complexes with 'star' and 'butterfly' topologies. Also families of heterometallic copper(II)/lanthanide(III) complexes with Schiff base ligands have been isolated with a structural core described as two cubanes sharing a common copper ion and general formula [Cu5Ln2(O2CMe)2(NO3)4(L)(HL)2] (LnIII = Gd, Tb, Dy) [Polyhedron, 2018, 150, 47-53, doi: 10.1016/j.poly.2018.05.003; Polyhedron, 2019, 169, 135-143, doi: 10.1016/j.poly.2019.05.004], heterometallic copper(II)/lanthanide(III) complexes with different structural core and general formula [Cu7Ln2(L)4(HL)2(piv)4(H2O)(MeOH)3] (LnIII = Gd, Tb, Dy) [Polyhedron, 2021, 195, 114960, doi: 10.1016/j.poly.2020.114960], and linear trinuclear heterometallic nickel(II)/lanthanide(III) complexes of general formula [Ni2Ln(H3L)4(O2CMe)2](NO3) (LnIII = Sm, Eu, Gd, Tb, Dy, Ho, Y) [Molecules, 2020, 25, 2280, doi: 10.3390/molecules25102280; Crystals, 2022, 12, 95, doi: 10.3390/cryst12010095]. All complexes were characterized by magnetic measurements, and spectroscopic techniques.
Coordination compounds based on pyridyl-oxime ligands
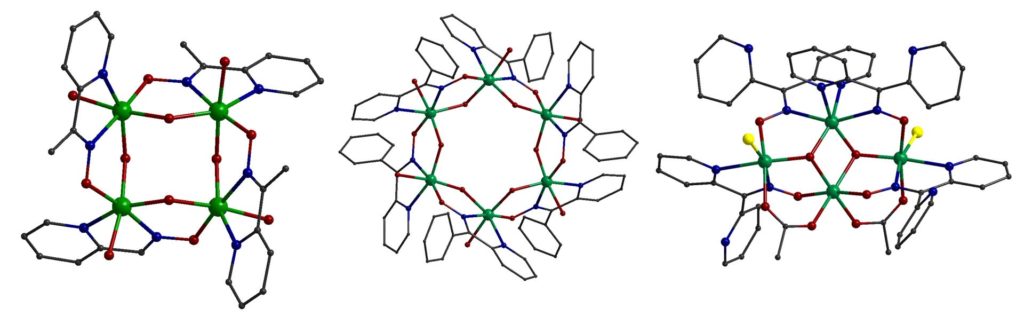
Pyridyl-oxime ligands of the general formula pyC(R)NOH (R = H, Me, Ph) gave triangular complexes containing the metal core [FeIII3(μ3-O)]7+, tetranuclear and hexanuclear inverse metallacrowns inv-{(OH)4[12-MCFeN(pyC(Me)NO)-4] and inv-{(OH)6[18-MCFeN(pyC(Me)NO)-6], tetranuclear complexes containing the ‘butterfly’ metal core [FeIII4(μ2-O)2]8+, and linear mixed-valence Fe(II/III) trinuclear and dinuclear complexes. Distorted [Fe4] squares, like the inv-{(OH)4[12-MCFeN(pyC(Me)NO)-4], with similar bridging are quite rare in 3d metal chemistry. All complexes were characterized by magnetic measurements, and spectroscopic techniques (Mössabuer, EPR). The complex [FeIII4O2Cl2(O2CMe)2{(py)2CNO}4] is the first example of a complex with ‘butterfly’ metal topology which possesses an S = 1 ground state because of the particular value of the Jbb/Jwb ratio [Inorg. Chem., 2006, 45, 7372-7381, doi: 10.1021/ic0604821; ChemSelect, 2016, 1, 147-156, doi: 10.1002/slct.201600037; Polyhedron, 2009, 28, 3221-3226, doi: 10.1016/j.poly.2009.05.016].
Coordination compounds based on pyridyl-ketone ligands
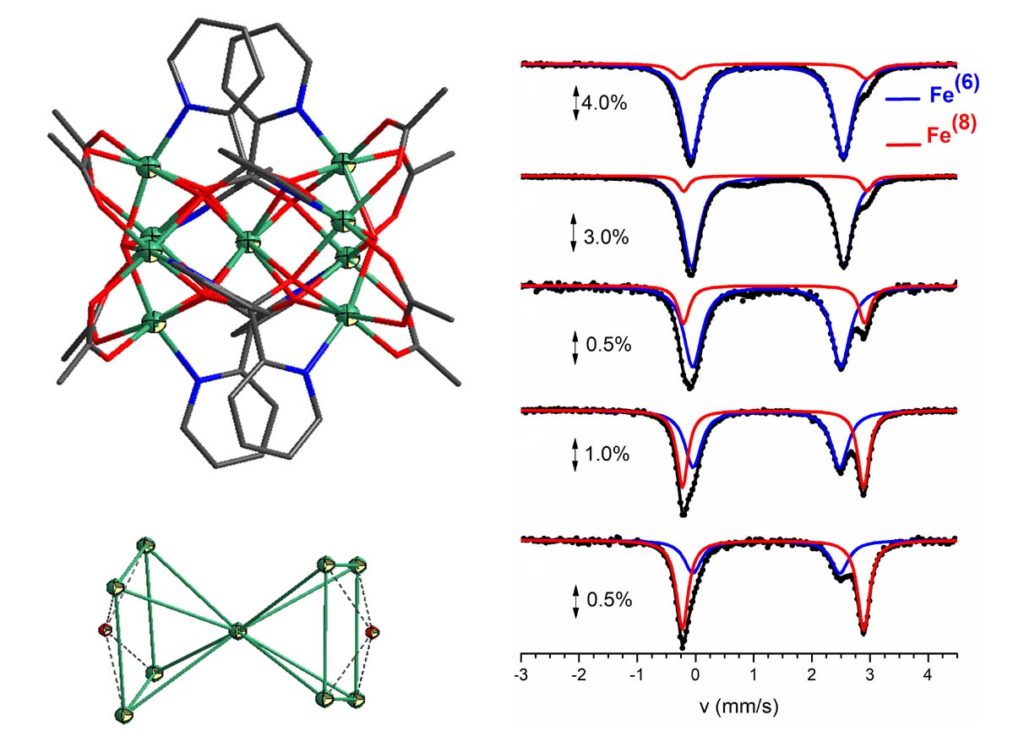
By using the ligand di-2-pyridyl ketone (py2CO, dpk) a family of heterometallic compounds with general formula [FeII9-xNiIIx(μ4-OH)2(O2CMe)8(py2CO2)4] (x = 1.00, 6.02, 7.46 and 7.81) was prepared. The synthesis of the compounds was performed under inert atmosphere in a MBraun Labmaster glovebox. All compounds are isomorphous and crystallize in the tetragonal space group P4/nnc. All compounds contain a central M(II) ion in an unusual 8-coordinate site and eight peripheral M(II) ions in distorted octahedral environments. The ligation between the metal ions is provided by four ligands in the gem-diol form, eight acetato ligands and two μ4-OH-. The topology of the nine metal ions is described as two square pyramids whish share a metal site with 8-coordination geometry. The metal ions in the base of each square pyramid are distorted octahedral and are bridged by a μ4-OH- group. The distribution of Fe(II) and Ni(II) ions over these two distinct coordination sites was established by a combination of X-ray fluorescence and Mössbauer spectroscopies, which showed that Fe(II) preferentially occupies the unique 8-coordinate metal site while Ni(II) accumulates in the octahedral holes. DFT calculations indicated that the distribution of ions across the two sites arises due to the large ligand field stabilization energy of the d8 Ni(II) ions in octahedral coordination, rather than preference of the d6 Fe(II) ions for 8-coordination [Dalton Trans., 2017, 46, 12835-12844, doi: 10.1039/C7DT02930F].
By using the ligand methyl-2-pyridyl ketone (pyCOMe) tetranuclear and trinuclear Fe(III) complexes were isolated, which contain the ligands pyCO(Me)CH=COpy and pyCO(Me)CH2CO(OMe)py. The new ligands were formed in situ from transformations of pyCOMe which involve aldol reaction-type mechanism for the formation of a new C-C bond. The compounds contain Fe(III) ions in octahedral and in square pyramidal coordination geometry. Mössbauer spectroscopy confirmed the two different metal sites. The complexes display slow relaxation of the magnetization and behave as SMMs.
Coordination polymers
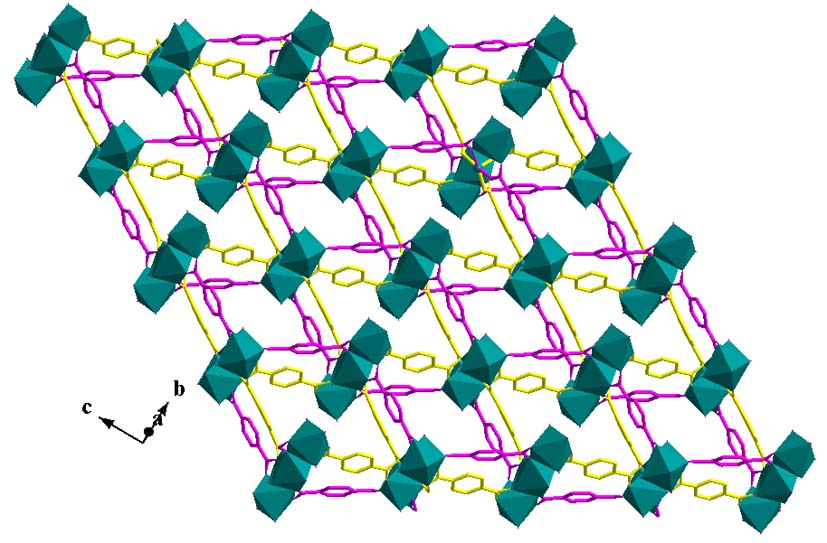
1D, 2D and 3D coordination polymers were synthesized by using aliphatic and aromatic dicarboxylic acids, monoamides of aliphatic dicarboxylic acids and flexible pyridyl-based ligands. For example 1D coordination polymers of copper(II) with maleic acid, fumaric acid, methyl-malonic acid, maleamic acid, and succinamic acid were prepared [Polyhedron, 2009, 28, 1085-1096, doi: 10.1016/j.poly.2009.01.023; Polyhedron, 2010, 29, 46-53, doi: 10.1016/j.poly.2009.05.075; Polyhedron, 2007, 26, 2536-2542, doi: 10.1016/j.poly.2006.12.033; Polyhedron, 2008, 27, 2131-2142, doi: 10.1016/j.poly.2008.04.005; Inorg.Chim.Acta, 2010, 363, 107-117, doi: 10.1016/j.ica.2009.09.039; Polyhedron, 2010, 29, 1870-1879, doi: 10.1016/j.poly.2010.03.003; Eur.J.Inorg.Chem., 2009, 4554-4563, doi: 10.1002/ejic.200900485]. The pyridyl-based ligand, 1,2-bis(4-pyridyl)ethane, is a neutral organic spacer which can exist in the gauche or anti conformation. This ligand gave 1D chains, [Cu(bpe)2(dmf)2]n(ClO4)2n.2ndmf, and 2D (4,4) wave-like sheets, [Cu(bpe)2(dmf)2]n(ClO4)2n.3.5ndmf, due to the conformational isomerism of bpe which exists in the gauche and anti form, respectively. 3D networks of different topology were also synthesized, i.e. [Cu2(bpe)4(NO3)2]n(NO3)2n.ndmf.3nH2O with a-Po network structure, and [Cu(bpe)2(NO3)2]n.2nH2O with inclined interpenetrating 2D (4,4) sheets. In all cases, the counteranions and the solvate molecules occupy the cavities of the networks [Polyhedron, 2011, 30, 963-970, doi: 10.1016/j.poly.2010.12.030].
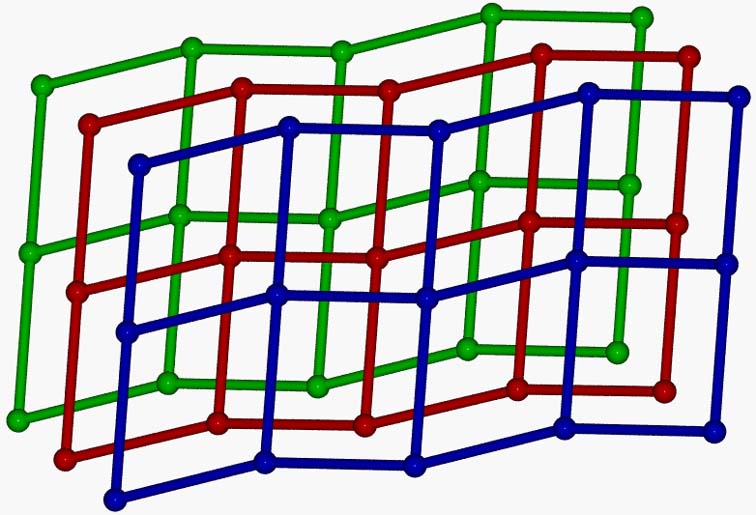
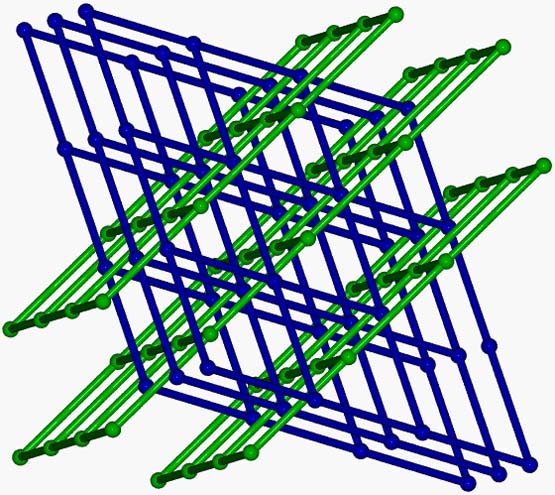
A 2D (4,4) network, [MnII4(ter)4(dmf)4(H2O)2]n.2ndmf (ter is the dianion of terephthalic acid) was synthesized, which consists of tetranuclear repeating units linked through the carboxylato groups of the ligands. The layers of the polymer are extended parallel to the bc crystallographic plane and are further associated to 3D supramolecular network through hydrogen bonding interactions. The magnetic susceptibility structures showed very weak exchange coupling between the Mn(II) ions within the tetranuclear repeating unit [Polyhedron, 2015, 85, 783-788, doi: 10.1016/j.poly.2014.10.011].