Chemical Mechanisms of Pharmaceuticals Synthesis
The synthesis of natural products and structurally complex pharmaceuticals may be challenging regarding the desirable stereospecificity and efficiency. Knowledge of the underlying chemical reaction mechanisms may provide suggestions in terms of reaction constituents and conditions in order to achieve the desirable results. However, since important aspects of reaction mechanisms cannot be always obtained by experiments alone, the concurrent theoretical quantum-mechanical investigation may provide the missing pieces of necessary information.
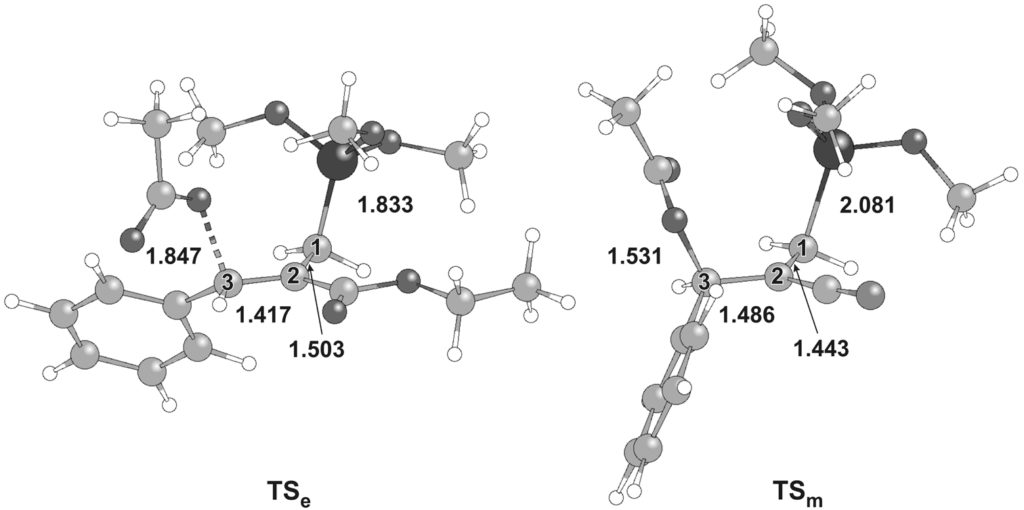
The reaction mechanism of the allylic substitution in Morita-Baylis-Hillman acetates (MBHAs) reacting with silyl phosphonites towards medicinally relevant β-carboxyphosphinic structural motifs was investigated to interpret the E/Z stereoselectivity difference between ester and nitrile reactants, in collaboration with the organic synthesis groups of Prof. D. Georgiadis in the Department of Chemistry, University of Athens and Dr. A. Papakyriakou in IBA, NCSR "D", (M. Kalyva, A.L. Zografos, E. Kapourani, E. Giambazolias, L. Devel, A. Papakyriakou, V. Dive, Y.G. Lazarou, D. Georgiadis, Chem. Eur. J. 2015, 21, 3278).