Microfluidics & Applications
Microtechnology allows the realization of miniaturized planar devices of micrometer-sized channels for performing small volume chemistry and biology, thus revolutionizing the life-sciences, medicine and diagnostics. Individual microfluidic components and devices, either as stand-alone or as parts of integrated lab-on-a-chip systems, have been designed, fabricated, and tested in our laboratories. Examples include digital microfluidics for electrowetting on dielectric-based transport of biomolecule-containing droplets in Si devices, PDMS-based microfluidic add-ons for sensors, microdevices for DNA amplification, micromixing, bacteria capturing, and DNA purification, on polymeric and/or printed circuit board (PCB) substrates. The group pioneered the implementation of flexible and rigid printed circuit board (PCB) approach that emerges as a very promising microfluidics manufacturing technology.
Electrowetting on dielectrics (EWOD) has gained considerable attention for its capacity of transporting smallest volumes of liquids especially in biochips and BioMEMS approaches. Optimized fluorocarbon films have been developed by our group as the hydrophobic top layer of droplet-based microfluidic devices, where droplet actuation and transport has been achieved via electrowetting on dielectric (EWOD). We have demonstrated droplet transport on an open microfluidic device with a series of sequentially activated microelectrodes.
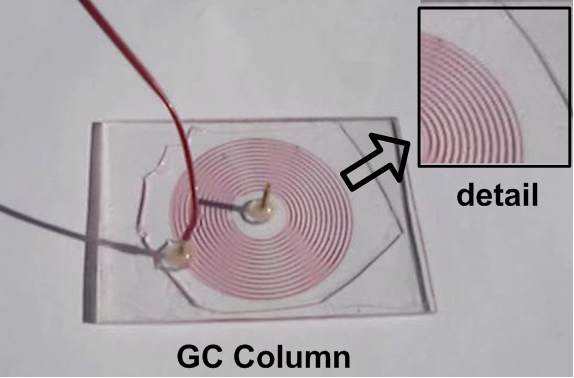
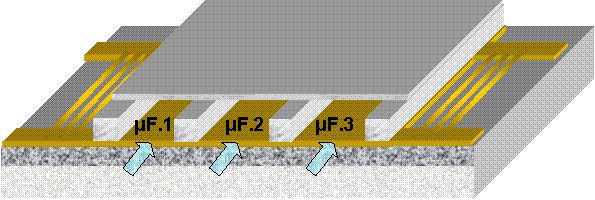
PDMS-based microfluidic components. Replica molding and rapid prototyping of PDMS-based components and devices has been adopted by our group for the fabrication of microfluidic modules and devices integrated with sensors, in collaboration with other groups. In particular, a gas chromatography microcolumn combined with a gas sensor has been shown to enhance the separation efficiency of the sensor. In addition, a multichannel microfluidic module has been integrated on a surface acoustic wave (SAW) sensor to allow for parallel biological multisample analysis (in collaboration with E. Gizeli, K. Mitsakakis, Univ. Crete & FORTH).
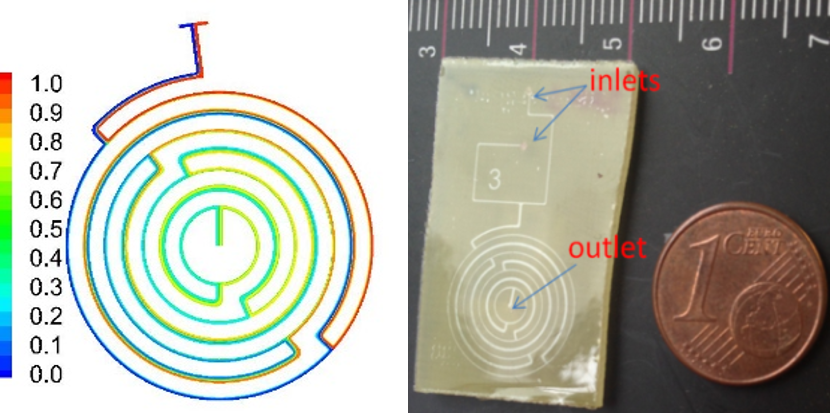
Microfluidics for chromatographic separations. Chromatographic microcolumns on Si or PMMA substrates have been developed by our group to separate phosphopeptides, in collaboration with Biomedical Research Foundation, Academy of Athens. The microcolumns consisted of 32 parallel microchannels with common input and output and were fabricated by direct lithography and plasma etching on Silicon and polymeric substrates, and sealed with a lamination film. TiO2-ZrO2 or TiO2 films, used as stationary phase in the affinity column, were deposited with rf magnetron sputtering, or with liquid phase deposition.
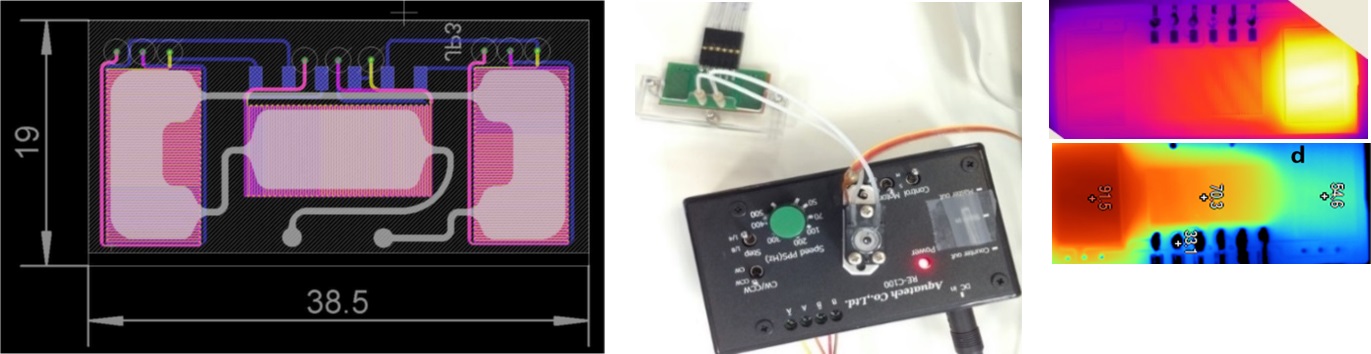
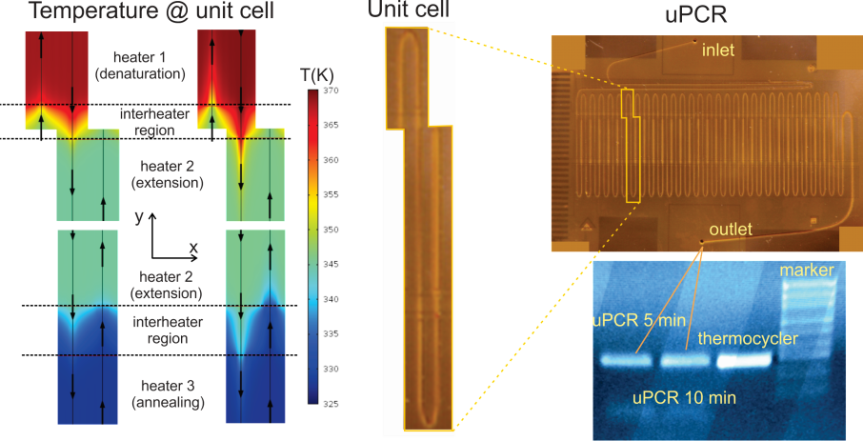
DNA amplification microdevices. Most molecular diagnostics rely on nucleic acid testing, with DNA amplification comprising an indispensable process with expanding applications in health, food safety and environmental monitoring. The most common DNA amplification method is the Polymerase Chain Reaction (PCR), which enables the detection of trace amounts of DNA within a biological sample. We have developed two generations of continuous flow microfluidic device for DNA amplification (μPCR), integrated with microresistors on commercially available substrates and processes compatible with the PCB industry. In the first generation of such devices, microchannels were formed on flexible polyimide (PI) substrates through the implementation of photopatternable layers by means of lithography. The small thermal mass of the chip, in combination with the low thermal diffusivity of PI substrate on which the heating elements reside, has yield an unprecedented low power consumption PCR chip with fast amplification rates (within few minutes compared to nearly 1 hour needed in conventional thermocyclers). In the second generation of continuous flow μPCR, commercially available, 4-layer printed circuit board (PCB) substrates have been employed, with in-house designed yet industrially manufactured embedded Cu micro-resistive heaters lying at very close distance from the microfluidic network. We demonstrated successful DNA amplification at total reaction times down to 2 min, with a power consumption of 2.7 W, rendering our μPCR device one of the fastest and lowest power-consuming devices, suitable for implementation in low-resource settings. Furthermore, we have demonstrated static well DNA amplification devices, implementing various amplification methods requiring either one single temperature (isothermal, i.e. HDA) or up to three different temperatures (thermocycled μPCR). In particular, based on our microfluidics-on-PCB concept, we have developed microfluidic devices that enable isothermal nucleic acid (DNA) amplification methods, such as Rolling Circle Amplification (RCA @ 65◦C), Helicase Dependent Amplification (HDA @ 65◦C), Loop Mediated Amplification (LAMP @ 65◦C) and Recombinase Polymerase Amplification (RPA @ 40◦C) on PCB substrates with embedded microresistors. In all such microdevices, the observed DNA amplification efficiency was comparable to that on conventional thermocyclers.
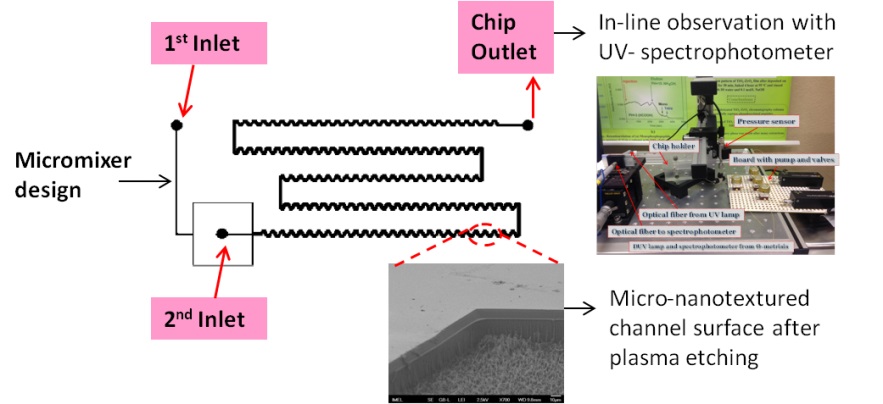
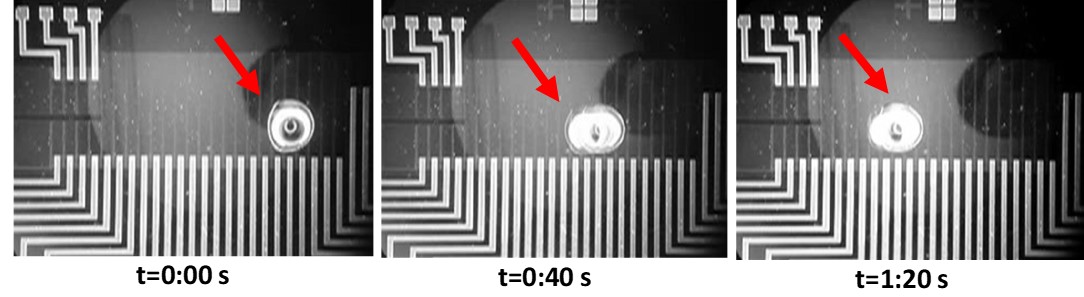
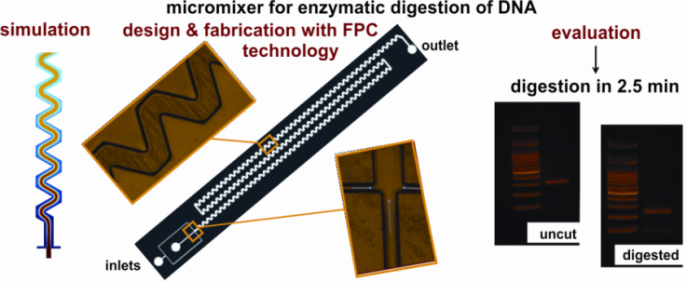
Micromixers. Mixing is imperative in microfluidics for the efficient performance of reactions in lab-on-chip systems. Passive micromixers (no external energy required) of various geometries have been designed, fabricated and demonstrated by our group. Examples include a micromixer with a zig-zag microchannel to perform simultaneously mixing of a restriction enzyme with DNA and digestion of DNA. When heated at 37 C, the micromixer achieved both complete mixing of the reagents and DNA digestion with restriction enzymes within 2.5 min. A novel, highly enhanced split and merge (SAM) passive micromixer has been also developed, with labyrinth-like microchannels to efficiently mix biomolecular solutions. Enzymatic digestion within 30 s was demonstrated.
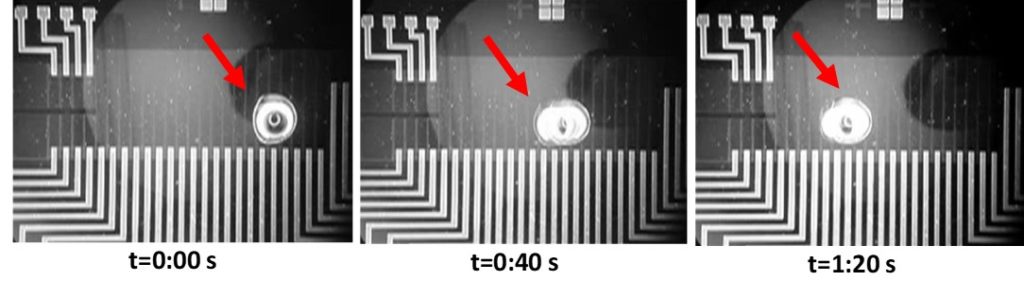
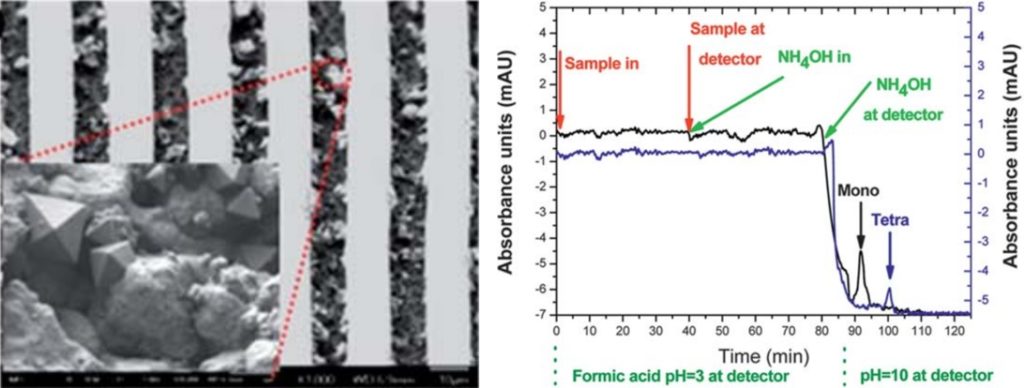
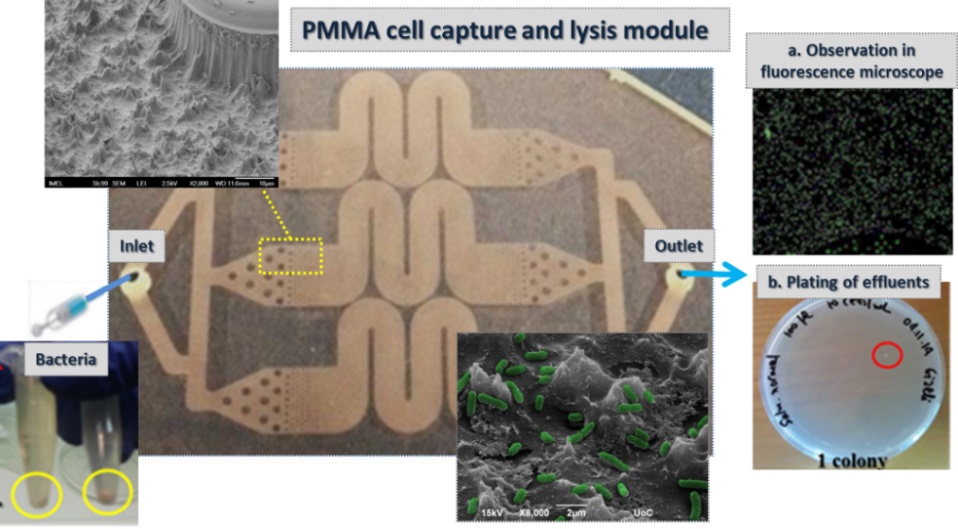
Microdevices for bacteria capturing. Sample preparation is indispensable functionality of most lab-on-chip devices for rapid pathogen detection at the point of need. We designed, fabricated, and demonstrated a novel sample preparation module comprising bacteria cell capture and thermal lysis on chip with potential applications in food sample pathogen analysis. Plasma nanotexturing of the polymeric substrate allowed enhancement of the surface area of the chip and the antibody binding capacity. The module exhibited 100% efficiency in Salmonella enterica serovar Typhimurium bacteria capture for cell suspensions below 105 cells/ml, and efficiency larger than 50% for 107 cells/ml. Moreover, thermal lysis achieved on chip from as low as 10 captured cells was demonstrated and was shown to compare well with off-chip lysis. Excellent selectivity (over 1:300) was obtained in a sample containing, in addition to S. Typhimurium, E-coli bacteria.
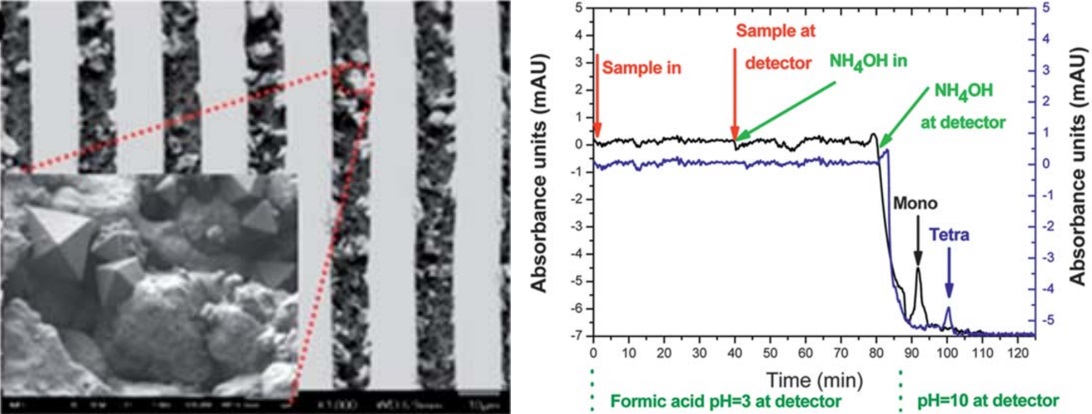
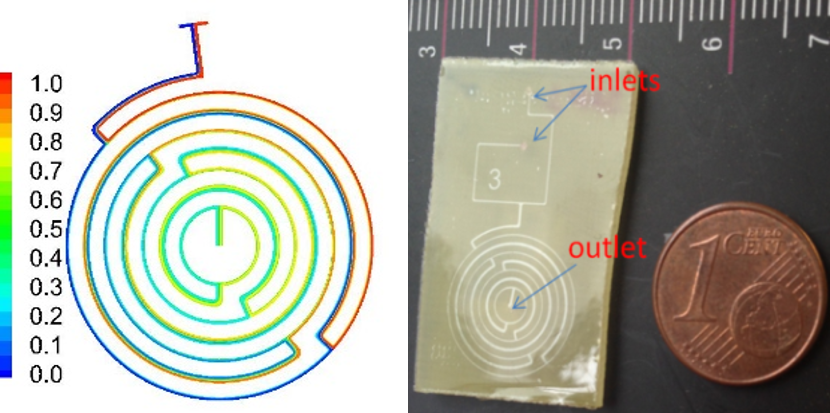
DNA purification microdevices. For molecular diagnostics applied in food industry, medicine, forensics and water industry, DNA purification is a procedure of high significance. A polymeric microfluidic chip capable of purifying DNA through solid phase extraction was designed, fabricated and evaluated in our group. Here again, plasma nanotextured surfaces were implemented to create high surface area as well as high density of carboxyl groups (eCOOH) able to bind DNA on the microchannel surface. The chip design incorporates a mixer so that sample and buffer can be efficiently mixed on chip under continuous flow. The chip was able to isolate DNA with high recovery efficiency (96± 11%) in an extremely large dynamic range of pre-purified Salmonella DNA as well as from Salmonella cell lysates that correspond to a range of 5 to 1.9 108 cells.