Molecular Magnetic Materials
Molecule based magnetic materials constitute an alternative approach towards the realization of novel systems with specific technological applications. Synthetic oligo- and poly-nuclear inorganic complexes of transition metals and lanthanides constitute an important class of these materials. The fields of applications include among others:
-Magnetic Storage
-Magnetic Cooling
-Sensors
-Quantum Computing
We are interested in determining the key factors that govern the behavior of these compounds. Our methodology is based on the application of spectroscopic techniques such as Electron Paramagnetic Resonance (EPR) and Mössbauer Spectroscopy as well as the application of static and dynamic magnetic measurements.
In the following we present characteristic examples from our activity:
- Determination of the oxidation and electronic state of the transition metal ions.
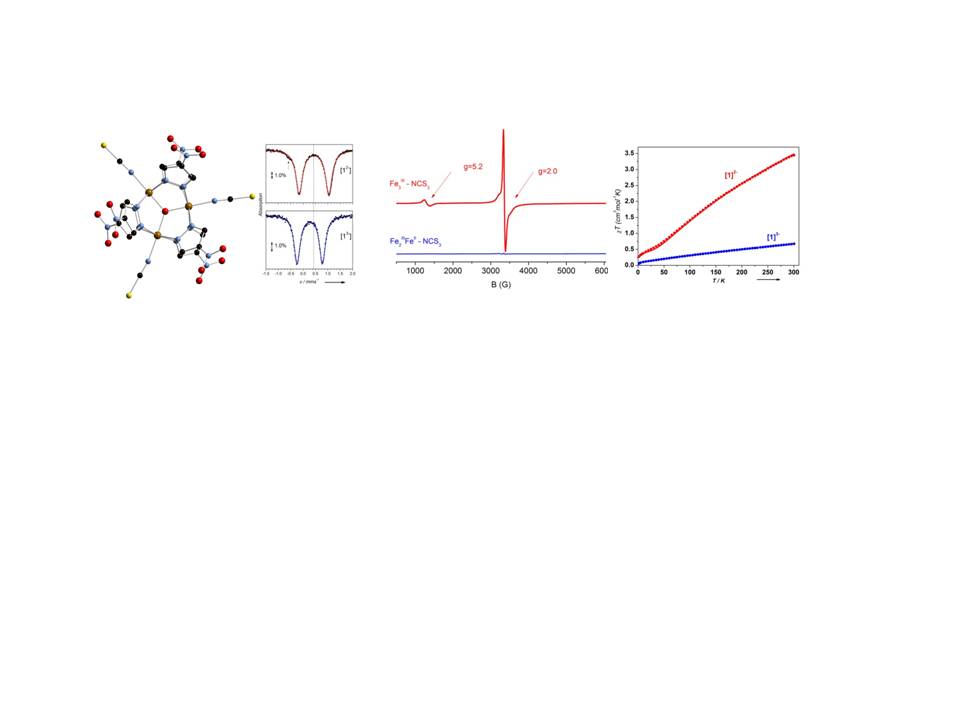
We studied the trinuclear ferric cluster [Fe3(μ3-O)(μ-4-NO2-pz)6(NCS)3]2–, [12–], The Mössbauer spectrum at 80 K gives rise to a quadruple doublet with parameters consistent with three equivalent high spin ferric ions in an octahedral environment comprising N/O ligands. Variable temperature magnetic susceptibility measurements indicate antiferromagnetic coupling. EPR spectroscopy at liquid helium temperatures gives rise to a strong EPR signal at g = 2.0 consistent with and S = 1/2 state. In the one electron reduced state the Mössbauer spectrum at 80 K comprises one doublet consistent with a fully delocalized system comprising two Fe3+(S=1/2) and one Fe2+(S=0) ions. EPR and magnetic susceptibility studies indicate that the mixed - valence trinuclear cluster is characterized by a diamagnetic ground state. The experimental observations are supported by theoretical calculations. Therefore, one-electron reduction of the Fe3(μ3-O) core induces a cascade wherein all three metal centers switch from high-spin Fe3+ to low-spin Fe2.66+ where the injected electron delocalizes over the three iron ions.
Ref: Angew. Chem. Int. Ed. 56, 582 (2017). doi: 10.1002/anie.201610534
-Dependence of spectroscopic parameters upon the coordination environment (symmetry, coordination number) of transition metal ions.
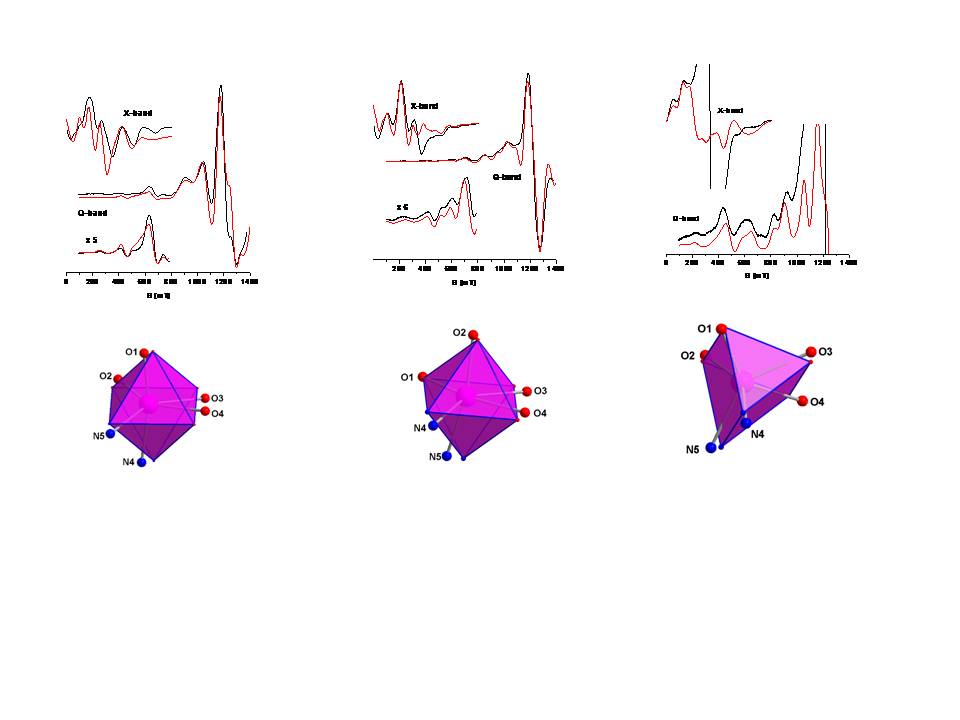
X- and Q-band EPR spectroscopy was applied to study the electronic properties of the [Mn(O,O)(N,N)(NO3)] complexes, (O,O) = [PhC(O)NP(O)PPh2]−, (N,N) = phenanthroline (1), neocuproine (2) and 2,2′-bipyridine (3). In 1 and 2 the Mn(II) ion is closer to an octahedral environment, whereas in 3 the symmetry is closer to trigonal prismatic. Analysis of the EPR spectra determined the zero-field splitting parameters of these S = 5/2 systems and revealed a small but significant difference in the magnitude of |D| for complex 3 compared to those of 1 and 2. These differences are attributed to the structural and electronic properties of complexes 1-3. The latter were probed by DFT calculations, which showed different DSOC contributions among the three complexes.
Ref: Polyhedron, 207, 115234 (2021). doi: 10.1016/j.poly.2021.115374
-Determination of the exchange coupling scheme in polynuclear transition metal clusters. The role of non isotropic exchange interactions in exchange coupled systems.
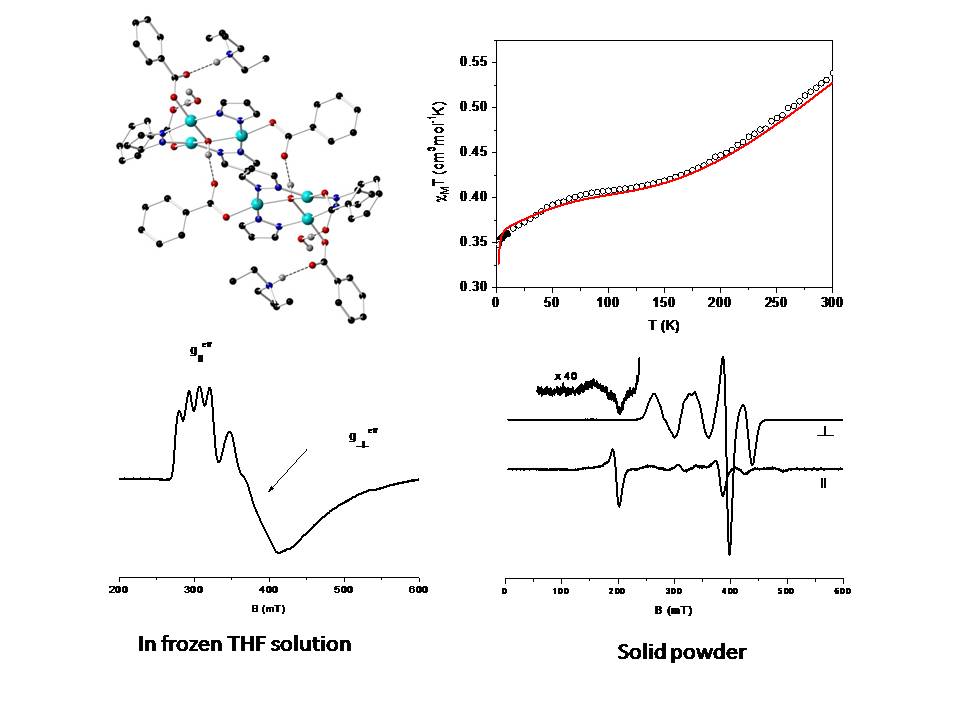
The complex [Cu3(μ3-OH)(μ-pz)3(PhCOO)3]_ (pz_ = pyrazolato anion) shows an isosceles triangular core, further forming a hexanuclear H-bonded aggregate. Analysis of variable temperature magnetic susceptibility data of a powder sample shows an antiferromagnetically-coupled Cu3-core with a doublet ground state. The fitting of magnetic data requires the inclusion of antisymmetric exchange, AE (HAE = GijSi x Sj). X-band EPR spectroscopy in a frozen tetrahydrofuran solution of the compound indicates isolated Cu3-species suggesting cleavage of the H-bonds in solution. The small value of g⊥,eff (<<2.0) is consistent with the presence of AE in agreement with the analysis of the magnetic measurements. EPR spectra were performed with powder samples of the cluster at liquid helium temperatures. The spectra are consistent with two interacting Sa,b = 1/2 species in the point dipolar approximation. From the analysis of the EPR spectra in the solid state an inter-spin distance of 4.4–4.5 Å was deduced which is very close to the distance between the Cu(1) and Cu(1)' sites of the two trimeric units as imposed crystallographically (4.3 Å).
Ref: Phys. Chem. Chem. Phys. 20, 17234 (2018). doi: 10.1039/c8cp02643b
-Spin relaxation properties of mononuclear transition metal complexes.
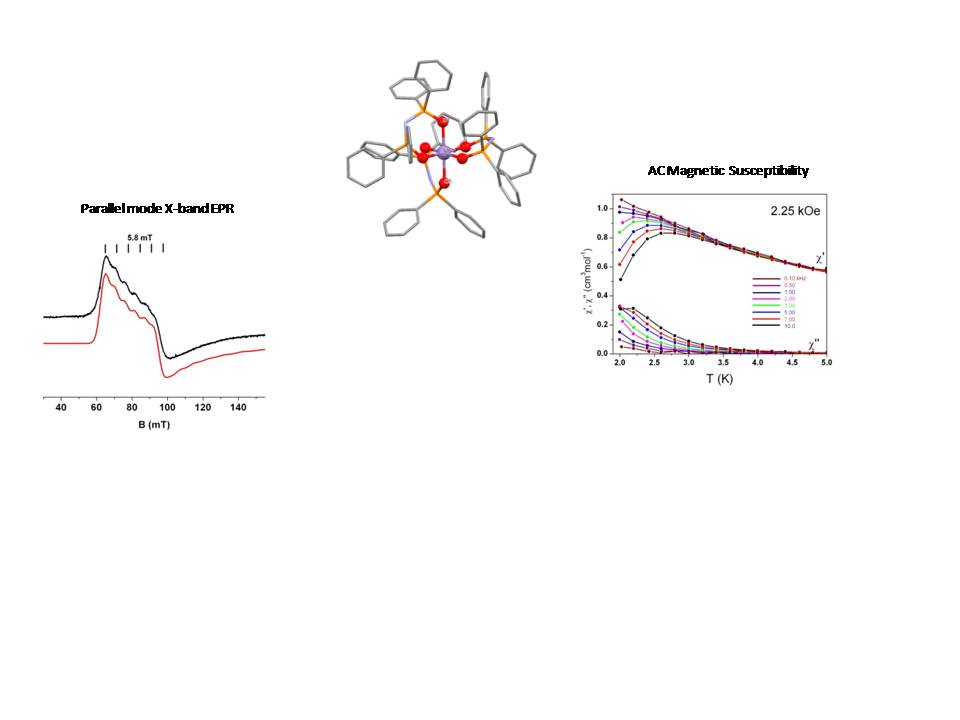
The spin relaxation properties of mononuclear transition metal and lanthanides ions complexes constitute an active field of research related to molecular magnetism in the last years. We studied the relaxation properties of the mononuclear complex, [Mn{(OPPh2)2N}3] where [(OPPh2)2N]— the tetraphenylimidodiphosphinato ligand. In this complex, the MnIII ion is found in a MnO6 coordination sphere exhibiting a Jahn-Teller distortion with an elongation axis. EPR spectroscopy (in the figure we show a representative EPR spectrum recorded at X-band in parallel mode) indicates that the complex is characterized by a negative zero field splitting parameter, D. Moreover, EPR spectroscopy implies the involvement of fourth order terms. The experimentally determined zero field parameters were in agreement with theoretical calculations. The compound was shown to exhibit slow spin relaxation in the presence of an external dc magnetic field. The compound was one of the first Mn(III) mononuclear complexes where the magnetic relaxation behavior was reported.
Refs:
a. Inorg. Chem. 52, 12869 (2013). doi: 10.1021/ic402042e
b. Inorg. Chem. 59, 13281 (2020). doi: 10.1021/acs.inorgchem.0c01636
-Spin relaxation properties of oligonuclear transition metal complexes.
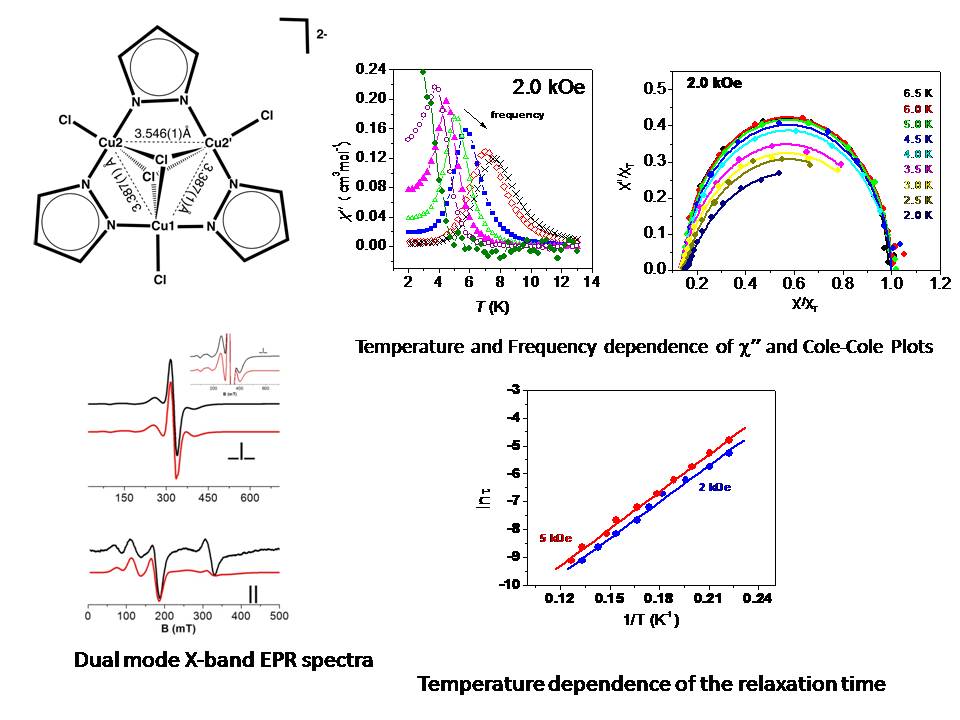
The trinuclear complex [Bu4N]2[Cu3(μ3-Cl)2(μ-pz)3Cl3] exhibits ferromagnetic interactions leading to an S = 3/2 ground state. EPR spectroscopy indicates that the ground state is characterized by a positive almost axial zero field splitting term of the order of 0.1 cm-1. No significant magnetic relaxation properties are expected due to the small spin value of the ground state and the negligible (and positive) zero field splitting parameter. Indeed, dynamic magnetic measurements in the absence of an external dc magnetic field indicate the absence of slow magnetic relaxation. However, a remarkable relaxation behavior is observed in the presence of an external dc magnetic field. The relaxation follows an Orbach mechanism with a barrier that relates to the S=3/2 ground and S = 1/2 excited manifolds.
Ref: Chem. Phys. Lett. 493, 185 (2010). doi: 10.1016/j.cplett.2010.05.011
-Electron hoping in mixed valence systems.
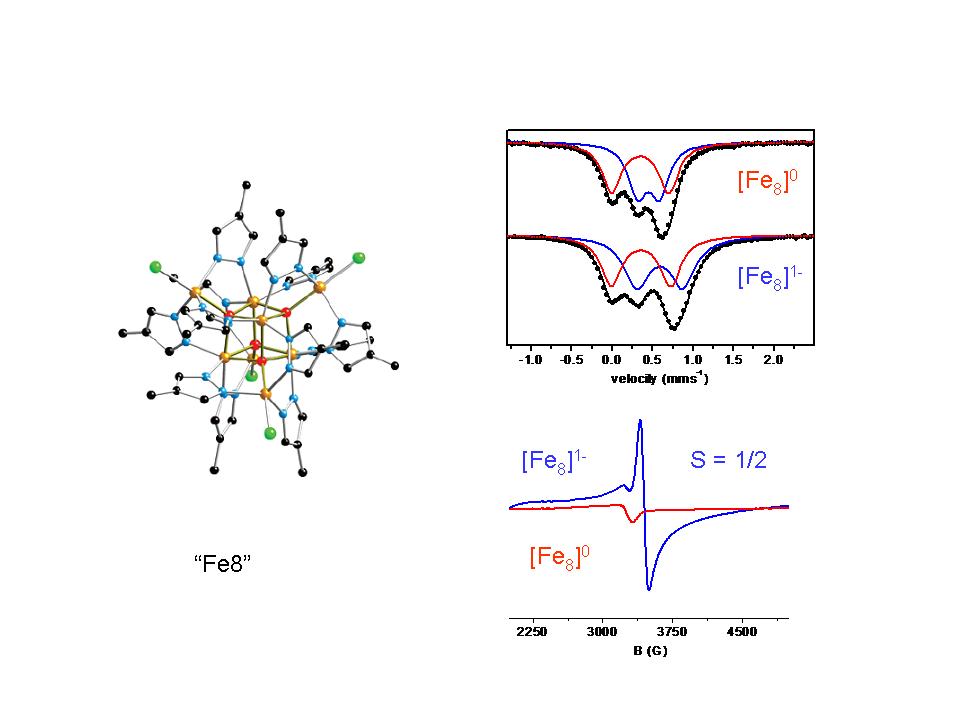
The clusters described by the general formula [Fe8(µ4-O)4(µ-4-R-pz)12X4] (pz = pyrazolato anion, C3H3N2–) (Fe8) contain a central Fe4(µ4-O)4-cubane surrounded by four additional Fe-centers. The terminal X- ligands are coordinated to the latter, forming a Fe8(µ4-O)4X4-core with tetrahedral geometry.The outer irons (Feo) have a trigonal bipyramidal coordination sphere whereas the FeIII centers in the cubane (Feo) have pseudo-octahedral geometries. The all ferric cluster exhibits a characteristic Mössbauer spectrum comprising two doublets the parameters of which reflect the different coordination environment (six vs five coordination). At this stage the cluster is EPR silent. Upon one electron reduction a cluster with nominal oxidation state Fe7IIIFeII is formed. Mössbauer spectroscopy indicates that the extra electron is delocalized over the iron sites of the cubane. At 4.2 K a characteristic EPR spectrum is revealed indicating that the ground state is characterized by S = 1/2.
Refs:
a. Inorg. Chem. 47, 11734 (2008). doi: 10.1021/ic801459s
b. Inorg. Chem. 50, 1021 (2011). doi: 10.1021/ic101691q
c. Dalton Trans. 43, 11269 (2014). doi: 10.1039/c4dt00020j
-Site occupation and preference in heterometallic clusters.
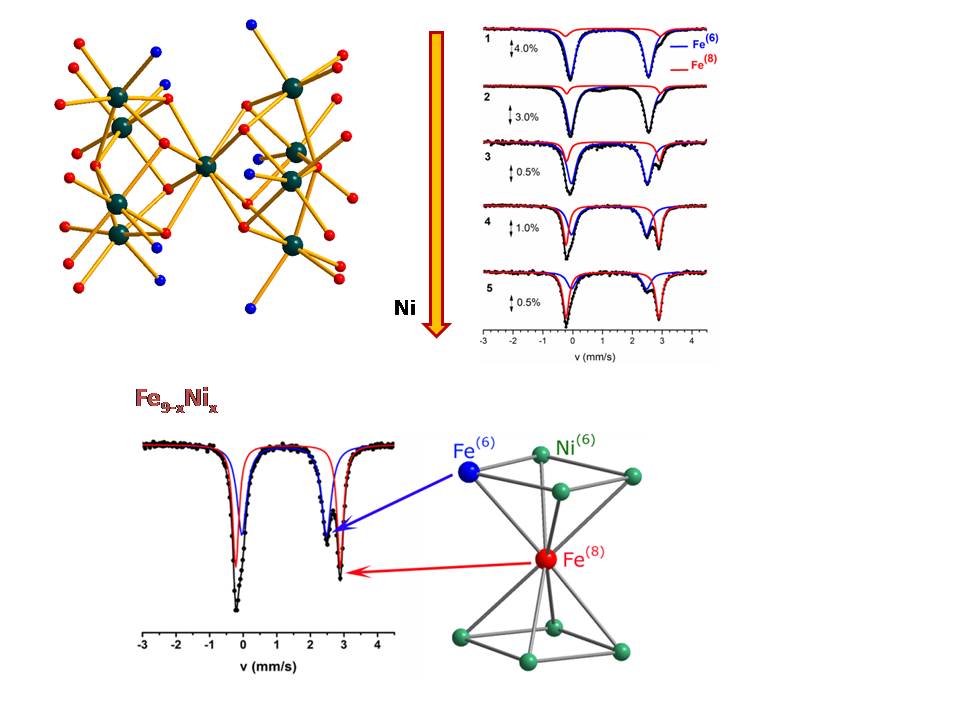
The reaction of mixtures of Fe(O2CMe)2∙2H2O and Ni(O2CMe)2∙4H2O of various compositions with di-2-pyridyl ketone afforded a family of hetero-metallic enneanuclear clusters with general formula [Fe9-xNix(µ4-OH)2(O2CMe)8(py2CO2)4] (Fe9-xNix; x = 0 - 8). All clusters contain a central MII ion in an unusual 8-coordinate site and eight peripheral MII ions in distorted octahedral environments. The all-iron complex exhibits a Mössbauer spectrum comprising two doublets at a 8:1 ratio with parameters that reflect the different coordination number of the ferrous ions: the majority species is attributed to the 6-coordinate ferrous ions whereas the minority species is assigned to the unique 8-coordinate site. A characteristic dependence on the NiII content of the ratio between the doublets is observed. With increasing Ni, the 8-coordinate ferrous doublet increases at the expense of the 6-coordinate ferrous doublet implying that as the Ni content increases Fe tends to occupy the 8-coordinate site. Theoretical calculations indicate that this behavior reflects the tendency of NiII ions to occupy octahedral sites. Site occupancy and preference are very common issues in inorganic mixed metal oxides and the present study shows that similar phenomena can be observed and studied in molecular clusters.
Refs: Dalton Trans. 46, 12835 (2017). doi: 10.1039/c7dt02930f