Materials for Batteries
The depletion of fossil fuels and the problem of greenhouse gas emissions is perhaps the biggest challenge of the coming decades. In order to achieve an energy-sustainable economy with reduced greenhouse gas emissions, it is imperative to look for other energy sources, storage methods and their use in daily activities. Alternative energy sources can be sought from wind, solar, geo-thermal and other renewable sources in combination with electric grid applications.
Li-ion batteries have some specific physicochemical characteristics (Li based batteries have the highest cell potential in comparison with any other element) such of high energy and power density making them important parts of portable electronics and electric vehicles industry, grid technologies. It is believed that the electric vehicles replace a large part of gasoline based transportation, then Li-ion batteries help to have a significant reduction of the greenhouse gas emissions.
The most common cathodic material in lithium ion batteries is the of lithium cobalt oxide while the anode is Li dispersed in graphite. This type of lithium batteries mostly used in portable electronic devices. The electrolyte is an organic compound.
Despite these perspectives cost issues, abundance, safety, rise several scientific challenges for Li-ion batteries. Therefore, Li-ion batteries are the subject of intense research interest.
The most commercial used cathodic materials are LiCoO2, LiNiO2, LiMnO2 and their mixed forms. The Co and Li, located in octahedral sites occupy alternating layers and form a hexagonal layer structure.
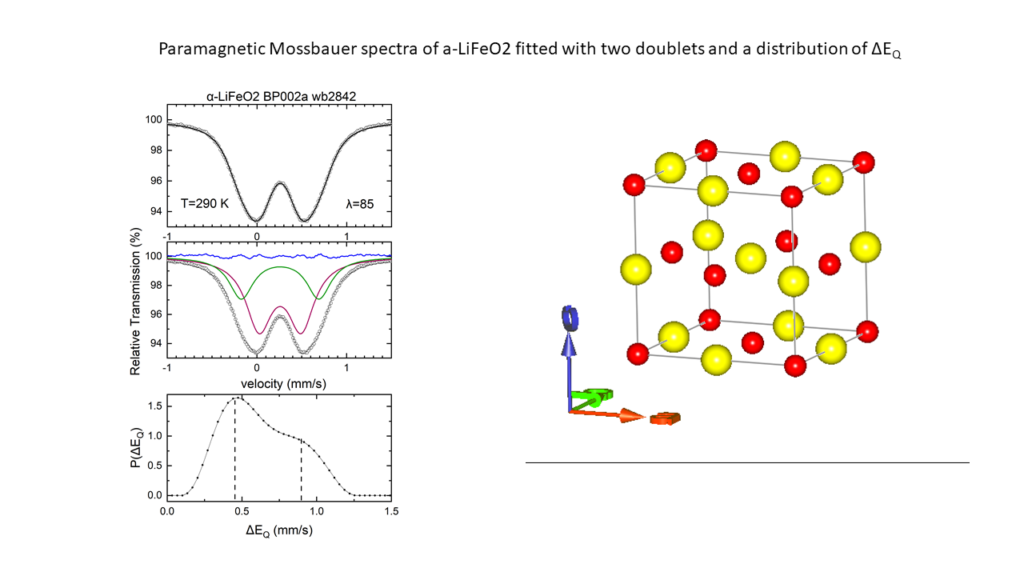
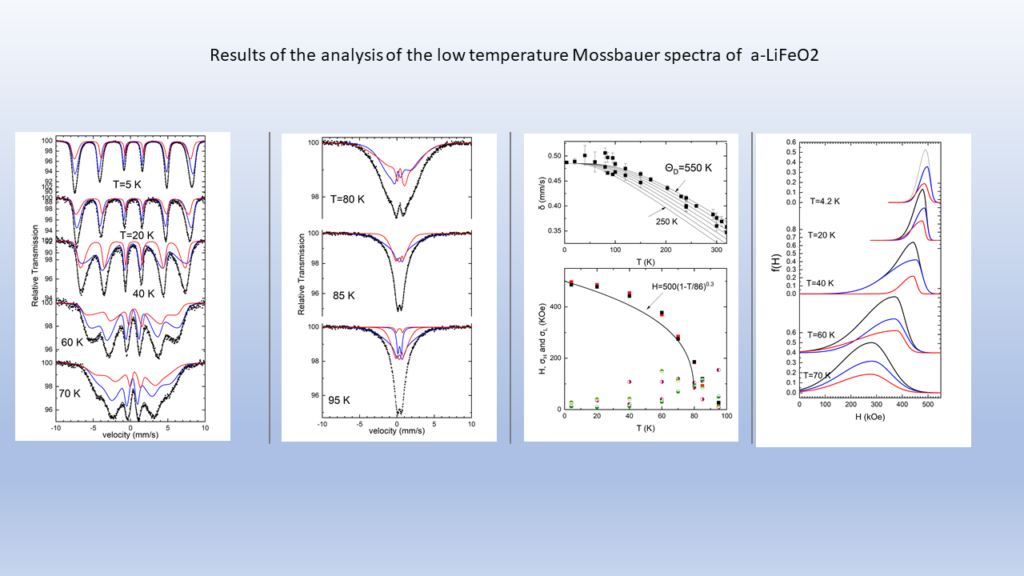
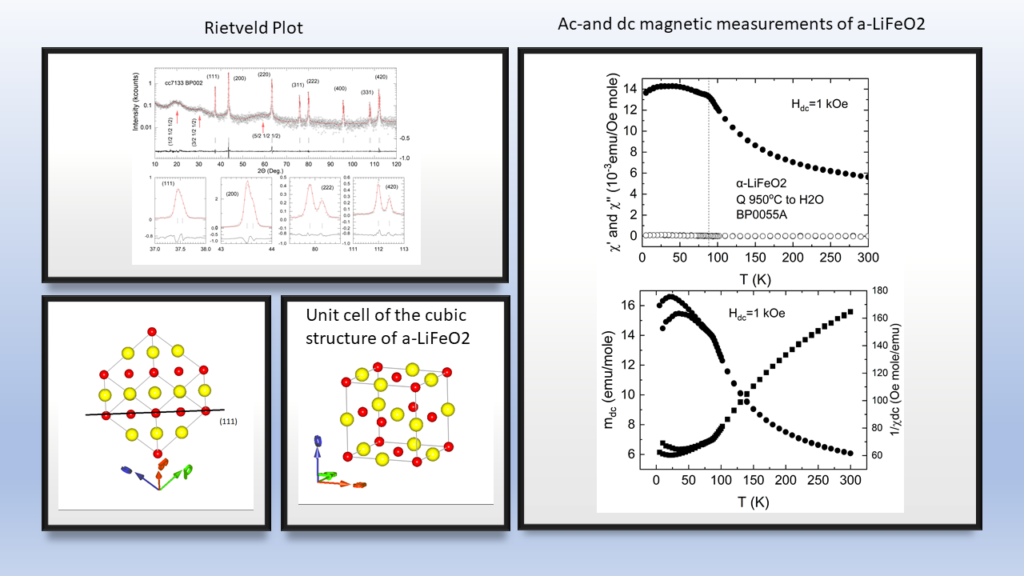
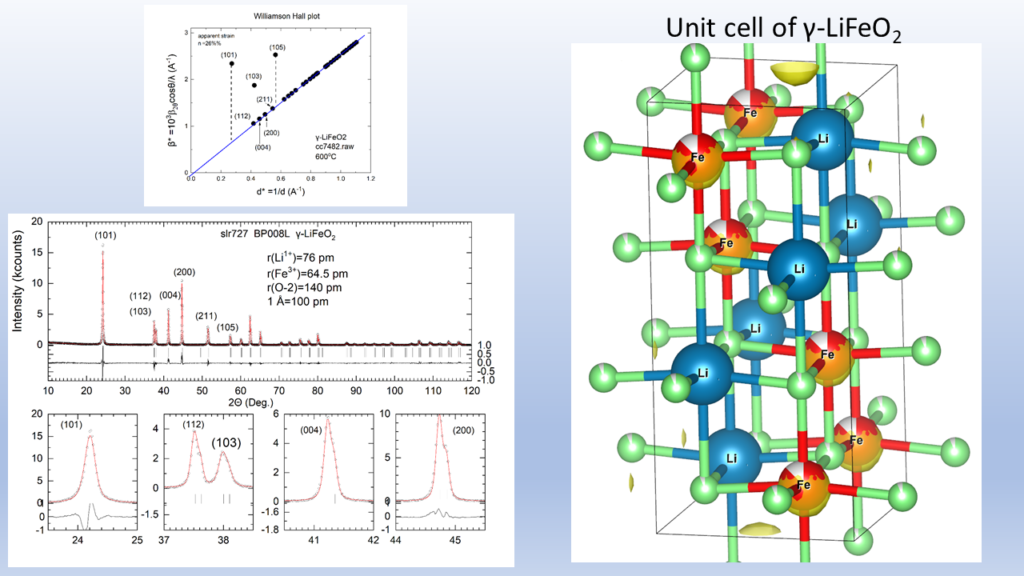
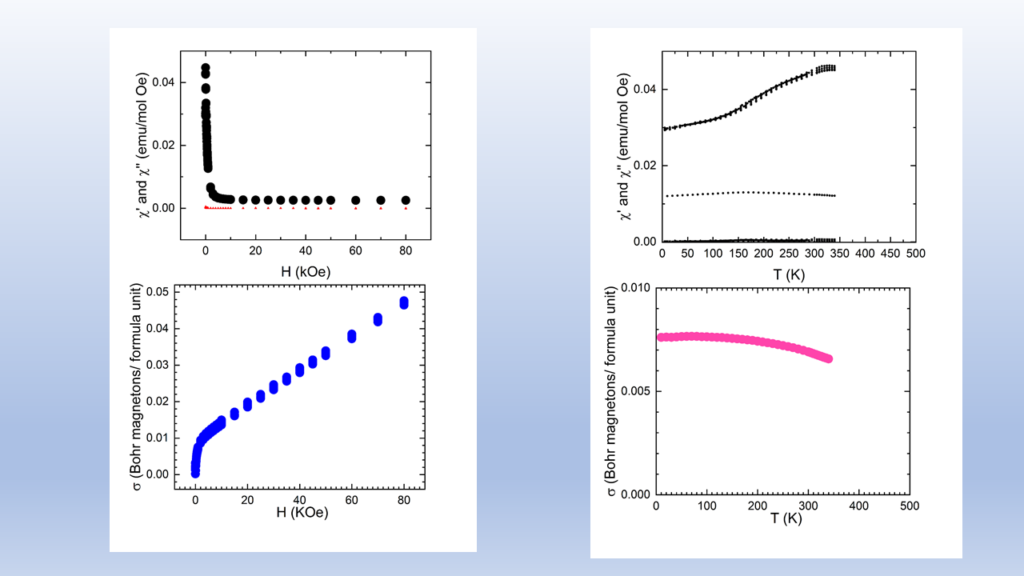
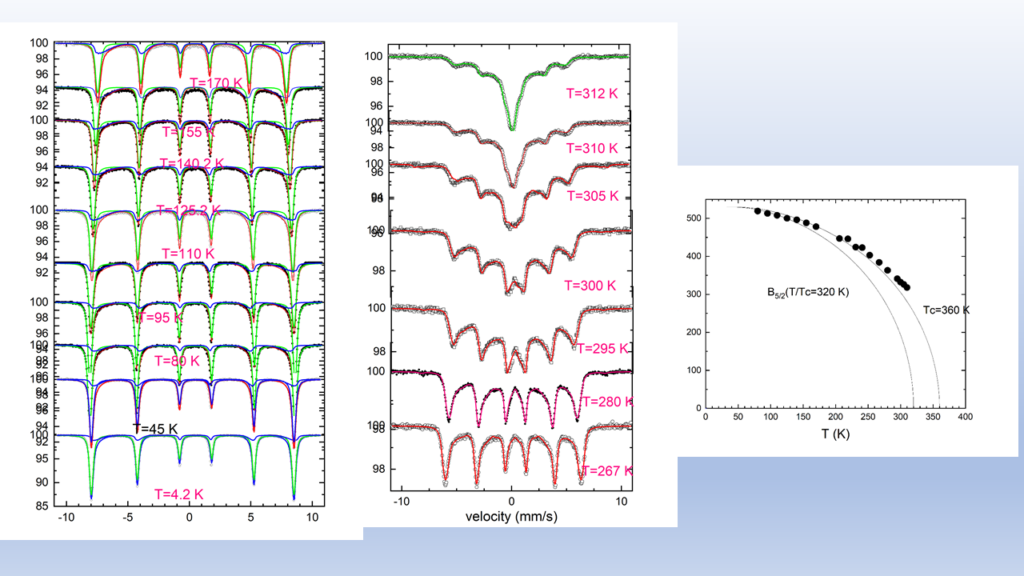
The high cost of cobalt and nickel combined with their low content, of the earth's crust, poses significant obstacles to the universal use of these elements in cathodes of lithium batteries.All of the above issues led us to the decision to dedicate research effort on the solid state chemistry and the study of the physical properties of lithium transition metal mixed oxides aiming to contribute to the understanding of the mobility of lithium but also to the discovery of new cheaper cathodic materials. In collaboration with the group of the program, “Crystallography and Coordination Chemistry of Materials”, “Molecular Magnetic and Bioinorganic Spectroscopy” of our Program “Magnetism and Superconductivity: Advanced Materials and Applications”, we have carry out an extensive research on LiFeO2 compound using x-ray diffraction data, Mossbauer spectra and magnetic measurements. Below shown are representative results regarding α-LiFeo2 (Fig1-3) and γ-LiFeO2 (fig-4-6).