Properties of polymer blends
The use of polymer mixtures is a widespread strategy to improve and/or combine the properties of the parent polymers. Blending of existing, well characterized polymers in order to meet the demand of new structures for specific applications is also economically advantageous in comparison to synthesis of new polymers. The properties of the blend, such as transport, mechanical and thermal properties, depend primarily on those of the parent homopolymers, the blend composition and the miscibility state of the blend.
Our work mainly concerns polymer blends for biomedical applications. Due to stringent safety specifications, research for polymeric materials for new biomedical uses is commonly focused on modification of existing polymers with proven safety and biocompatibility. We focus on
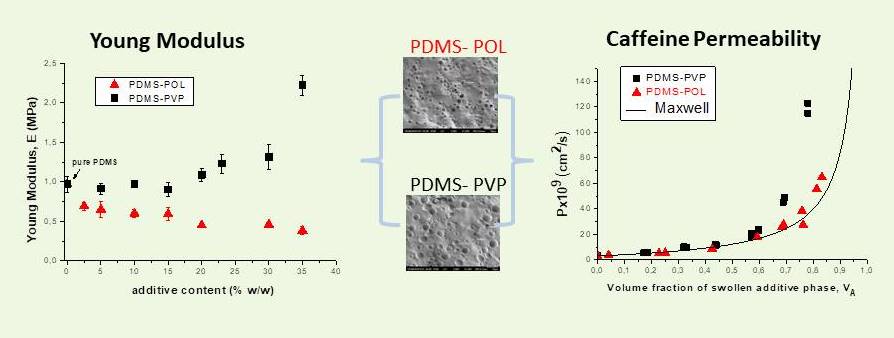
(i) Modification of hydrophobic elastomers through blending with hydrophilic polymers in order to enhance the affinity of the former to water. The resulting increase in the permeability of water vapor and of bioactive substances (drugs, proteins) is sought in controlled release systems, wound dressings, transdermal patches, etc. The elastomer–hydrophilic polymer blends possess a two-phase morphology due to the immiscibility of its components.Detailed work has been on blends of PDMS, a widely used thermosetting polymer for biomedical uses, with hydrophilic polymers, namely PEG, PVP, Poloxamer.1,2 In each system the increase in permeability of model polar drugs, with increasing additive content is studied in relation to the water uptake of the blend, the morphology and the mechanical properties of the system, and the cure characteristics of PDMS, that are affected by the presence of the additive. It was shown that the interrelation of all these properties in a particular PDMS-additive system defines the range of additive content that ensures a gradual and thus controllable increase of permeability. The upper limit of this range defines the onset of some form of continuity of the additive phase which also denotes the deviation from the Maxwell’s formula for two-phase systems, assuming that the dispersed phase is the swollen additive phase.
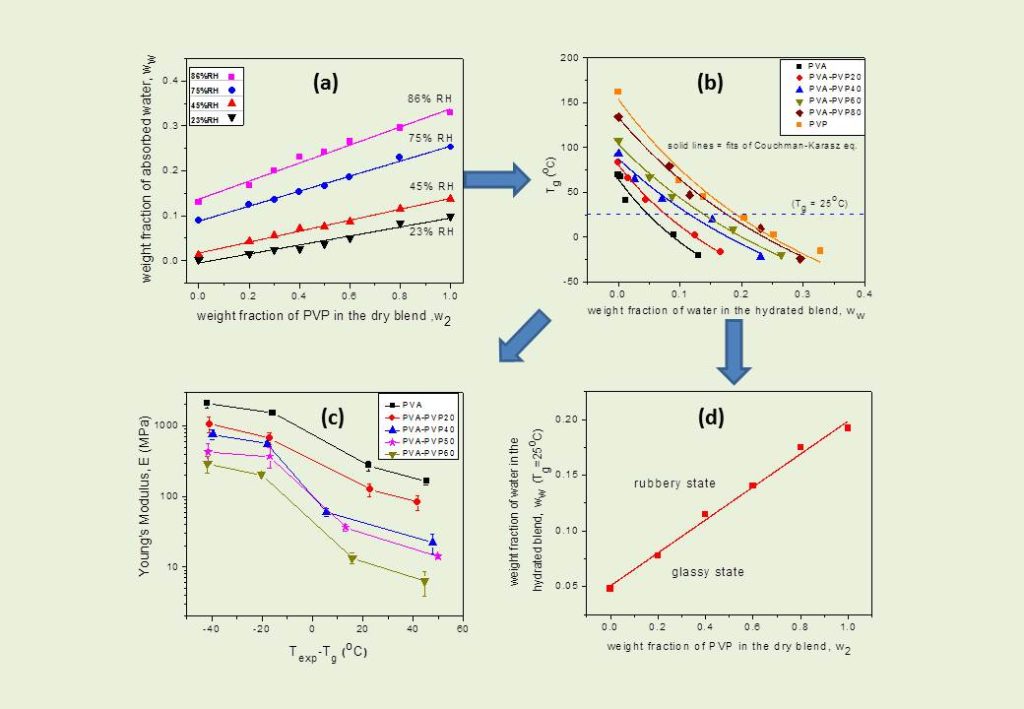
(ii) Blends of miscible hydrophilic polymers. In this case, due to the hydrophilicity of both parent polymers, blends absorb readily water when exposed to external humidity. The plasticizing effect of even small amounts of sorbed water results in depression of the glass transition temperature of the blend and affects the material’s performance, especially in applications such as functional films and protective coatings. Eventually with increasing humidity and amount of sorbed water, a point is reached where the glass to rubbery transition occurs at ambient temperatures (see Fig b). The water content of each blend necessary to reach this point is of critical importance for the performance of the material at practical external conditions, as many properties undergo a drastic change at that point (e.g. permeation, diffusion and rheological properties, and mechanical strength, stiffness and ductility (see Fig c).
Currently we focus on the effect of external humidity level on the degree of hydration of hydrophilic blends, the concurrent impact of hydration on thermal and mechanical properties and the establishment of inter-property relations.
Selected References
- A.I. Panou, N. Mantes, K.G. Papadokostaki, M. Sanopoulou, “Solute permeability of elastomeric films containing dispersed swellable hydrophilic particles: A composite material approach”, Eur. Polymer J. 93 (2017) 471–479
- M. Tsoka, P. Oikonomou, K. G. Papadokostaki, and M. Sanopoulou “Properties of Polydimethylsiloxane Modified by Blending with Polyvinylpyrrolidone and a Poly(ethylene oxide)-Poly(propylene oxide) Triblock Copolymer, Ind. Eng. Chem. Res.59 (2020) 5797−5807
- P. Oikonomou, M. Sanopoulou, K. G. Papadokostaki “Blends of Poly(vinyl alcohol) and poly(vinyl pyrrolidone): Interrelation between degree of hydration and thermal and mechanical properties” Ind. Eng. Chem. Res. 60 (2021) 14201-14212