Cassane-type furanoditerpenoids
Stemming from the interest of our group in the synthesis and study of natural products with important biological activities and following past accomplishments in this area (e.g., total synthesis of Scyphostatin1 (1, Fig. 1), a potent and selective N-SMase inhibitor; the first enantioselective total synthesis of Laurenditerpenol2 (2, Fig. 1), a marine diterpene that targets hypoxic signalling in tumour cells), a research project focusing on the natural product Taepeenin D (3, Fig. 2) has been initiated.
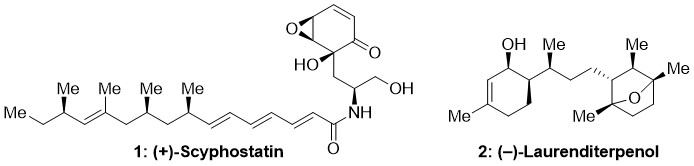
Figure 1. Chemical structures of Scyphostatin (1) and Laurenditerpenol (2).
Taepeenin D (3) is a cassane-type diterpenoid originally isolated from Caesalpinia crista that has been identified as a constituent of Acacia pennata with significant Hh/Gli-mediated transcription inhibitory activity (IC50 = 1.6 µM) and selective cytotoxicity against cancer cells with increased Hh signalling levels (IC50 = 3.2 – 3.4 µM).3 The Hedgehog (Hh) signalling pathway is one of the pathways that control embryonic patterning and cellular proliferation and differentiation. However, its abnormal activation has been linked with the occurrence of basal cell carcinoma and meduloblastoma while several other tumours (such as cancers of the skin, brain, lung, pancreas, digestive tract, prostate) are co-dependent on Hh signalling. Moreover, recent evidence suggests that Hh signalling is important for the self-renewal of cancer stem cells in pancreatic cancer, glioblastoma, multiple myeloma as well as in chronic myeloid leukemia. Thus, inhibition of Hh pathway has become an attractive strategy in anticancer therapy and several related clinical trials are underway.4
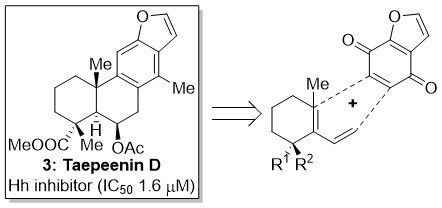
Figure 2. Chemical structure of Taepeenin D (3) and strategic retrosynthetic disconnection.
Several Taepeenin D-related analogues (4, Fig. 3) were prepared exploiting abietic acid (5, Fig. 3), a readily available starting material; main constituent of rosin. Evaluation of their activity as Hh/Gli inhibitors allowed the identification of important structural features (SAR studies) and the identification of new leads.5 In parallel, the total synthesis of this natural product was pursued. Based on a novel hexafluoroisopropanol mediated Diels–Alder reaction (Fig. 2) the enantioselective total synthesis of several cassane-type diterpenoids has been accomplished.6 Efforts to complete the first total synthesis of Taepeenin D and related cassane-type diterpenoids are ongoing.
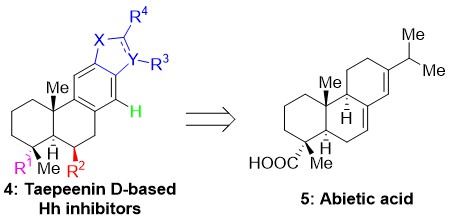
Figure 3. Structures of Taepeenin D-related analogues (4) and abietic acid (5).
References
- a) E.N. Pitsinos, A. Cruz, Org. Lett. 2005, 7, 2245–2248; b) E.N. Pitsinos, N. Athinaios, Z. Xu, G. Wang, E.-I. Negishi, Chem. Commun. 2010, 46, 2200–2202.
- E.N. Pitsinos, N. Athinaios, V.P. Vidali, Org. Lett. 2012, 14, 4666–4669.
- a) S. Cheenpracha, R. Srisuwan, C. Karalai, C. Ponglimanont, S. Chantrapromma, K. Chantrapromma, H. K. Fun, S. Anjum, R. Atta ur, Tetrahedron 2005, 61, 8656–8662; b) Y. Rifai, M. A. Arai, T. Koyano, T. Kowithayakorn, M. Ishibashi, J. Nat. Prod. 2010, 73, 995–997.
- For related reviews see: a) T. N. Trinh, E. A. McLaughlin, C. P. Gordon, A. McCluskey, MedChemComm 2014, 5, 117–133; b) F. Ghirga, M. Mori, P. Infante, Bioorg. Med. Chem. Lett. 2018, 28, 3131–3140; c) M. Xin, X. Ji, L. K. De La Cruz, S. Thareja, B. Wang, Med. Res. Rev. 2018, 38, 870–913; d) I. Galperin, L. Dempwolff, W. E. Diederich, M. Lauth, J. Med. Chem. 2019, 62, 8392–8411.
- a) M. Chatzopoulou, A. Antoniou, E. N. Pitsinos, M. Bantzi, S. D. Koulocheri, S. A. Haroutounian, A. Giannis, Org. Lett. 2014, 16, 3344–3347; b) A. Antoniou, M. Chatzopoulou, M. Bantzi, C. M. Athanassopoulos, A. Giannis, E. N. Pitsinos, MedChemComm 2016, 7, 2328–2331.
- a) E. Tzouma, I. Mavridis, V. P. Vidali, E. N. Pitsinos, Tetrahedron Lett. 2016, 57, 3643–3647; b) E. N. Pitsinos, I. Mavridis, E. Tzouma, V. P. Vidali, Eur. J. Org. Chem. 2020, 4730−4742.