Polyprenylated acylphloroglucinols
Prenylated acyl phloroglucinols constitute an exciting class of natural compounds with various biological activities. They are isolated from different plant species (e.g., the genus Hypericum (Guttiferae) and Humulus lupulus). Complex secondary metabolites, like the type A polyprenylated polycyclic acyl phloroglucinol (PPAP) hyperforin but also simpler naturally occurring prenylated acylphloroglucinol derivatives such as bitter acids (e.g., lupulone, colupulone, humulone), are widely known for their use in traditional medicine and food industry as they exhibit antimicrobial, antioxidant and anticancer activity.1
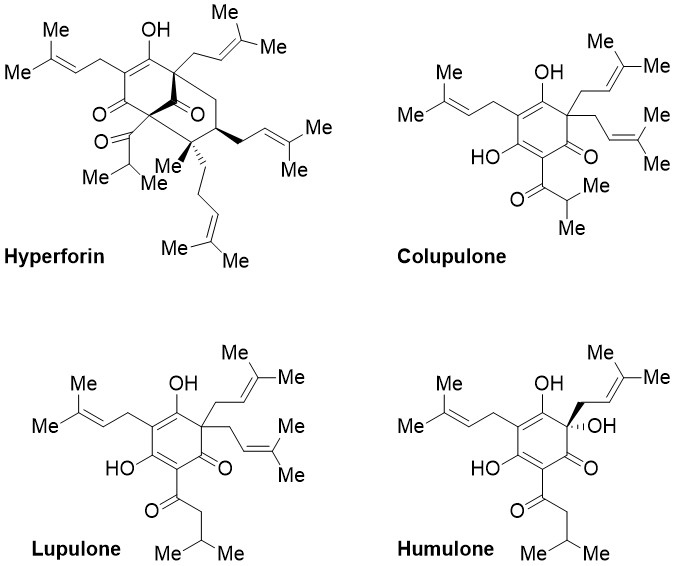
Figure 1. Prenylated acyl phloroglucinols.
Intrigued by the fascinating structures of PPAPs and the biological activities of bitter acids, we have been involved in synthetic studies towards these molecules aiming to explore further their chemistry and biological properties. We have developed a biomimetic synthesis of the polycarbocyclic skeleton of hyperforin2 (I, Figure 2) and discovered fascinating transformations to the bioactive compound II.3
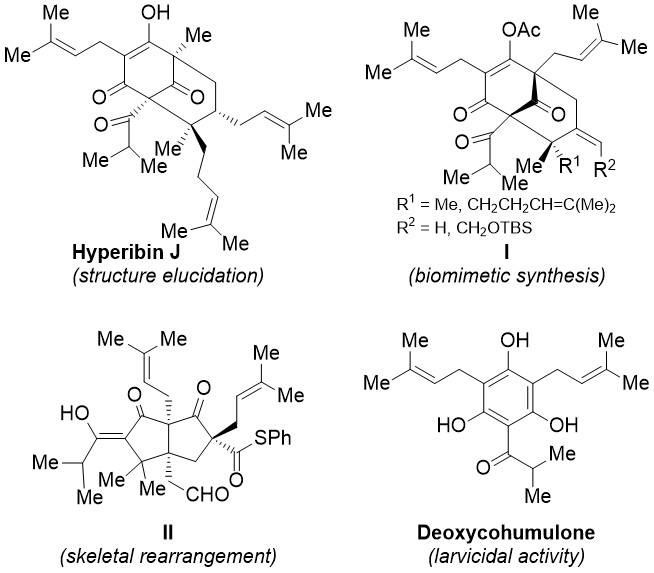
Figure 2. Activity's results to date.
In addition, we have elucidated the structure of the natural product hyperibin J, revising the proposed structure of adhyperfirin.4 Moreover, we have evaluated hyperforin and deoxycohumulone as larvicidal agents against the mosquito Culex pipiens with encouraging results.5 As part of this ongoing research, improved natural and novel larvicidal agents have been developed.6 The above studies have been the objective of two PhD theses and an MSc thesis implemented in our laboratory in collaboration with the Agricultural University of Athens and the Benaki Phytopathological Institute in Greece.
References
- (a) X.-W. Yang, R. B. Grossman, G. Xu, Chemical Reviews 2018, 118, 3508–3558; (b) G. Astray, P. Gullon, B. Gullon,P. E. S. Munekata, J. M. Lorenzo, Appl. Sci. 2020, 10, 5074–5092.
- E. A. Couladouros, M. Dakanali, K. D. Demadis, V. P. Vidali, Org. Lett. 2009, 11, 4430–4433.
- V. P. VIdali, K.P. Mitsopoulou, M. Dakanali, K. D. Demadis, A. D. Odysseos, Y. A. Christou, E. A. Couladouros, Org. Lett. 2013, 15, 5404–5407.
- K. P. Mitsopoulou, V. P. Vidali, A. Maranti, E. A. Couladouros, Eur. J. Org. Chem. 2015, 287–290.
- Apostolia Makri, MSc thesis: “Synthesis of acylphloroglucinol derivatives and assessment of their toxicity against larvae of the species Culex pipiens L. (Diptera: Culicidae)”, January 2020, Agricultural University of Athens.