Biocompatible Complexes of Pharmaceutical Compounds
Natural and synthetic cyclodextrins (CDs) comprise a class of versatile carbohydrates in pharmaceutical industry. Their hydrophobic cavity combined with a chemically modifiable exterior enables the encapsulation of suitably sized lipophilic molecules or molecular groups. Therefore, CDs may serve as tailor-made carriers in a variety of medicinal applications. In this activity, theoretical calculations are employed to determine the structural and thermochemical aspects of host CDs as well as their interactions with guest molecules in aqueous solution, in collaboration with the experimental research group of Dr. K. Yannakopoulou in INN.
The calculations of molecular properties for CDs and their host-guest complexes are performed at semi-empirical levels of theory including the implicit treatment of solvation by the conductor-like screening model (COSMO). Two typical cases of molecular systems investigated in this activity are presented below.
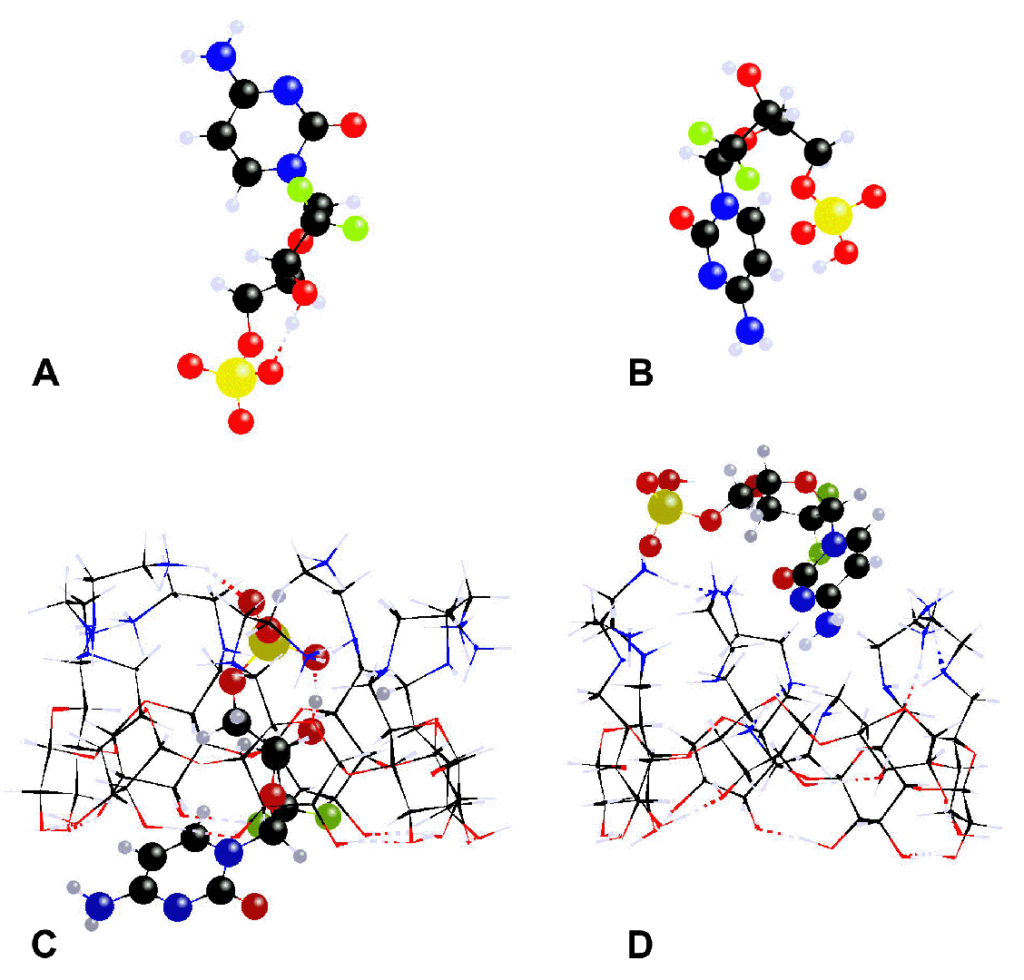
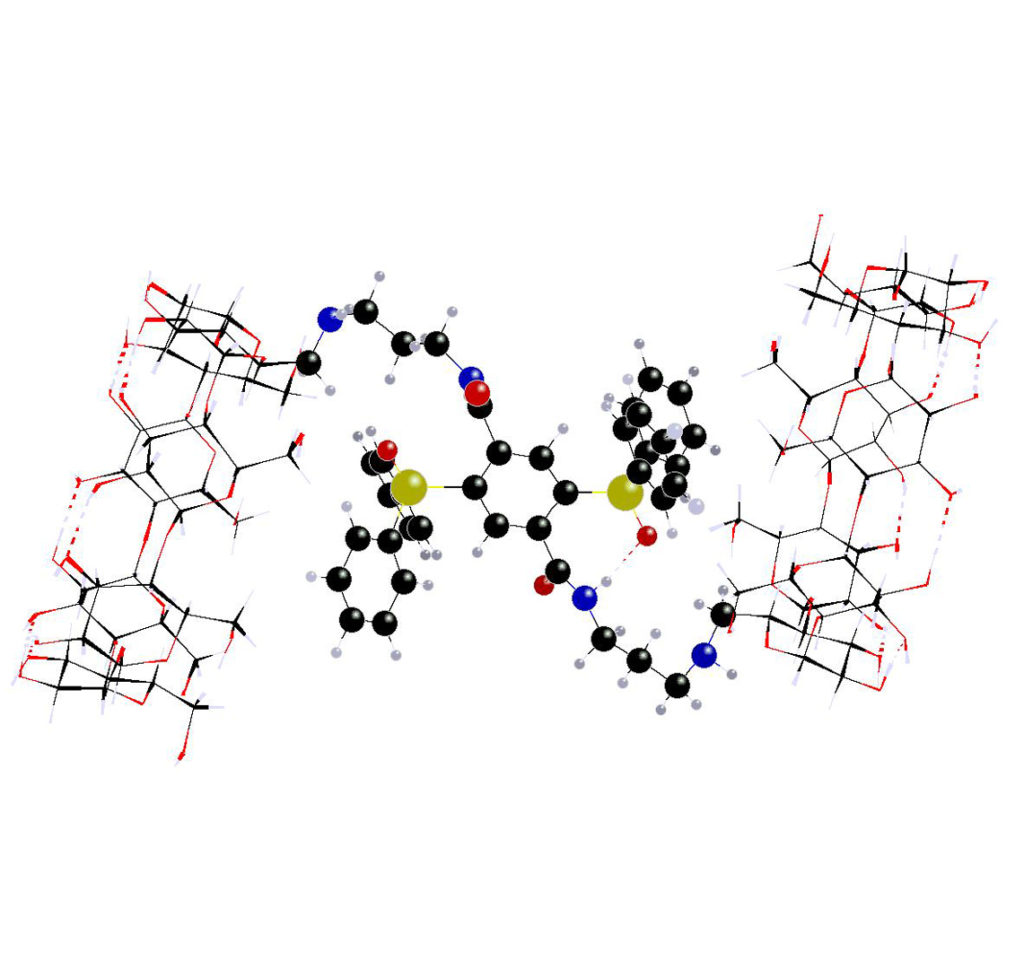
1) Bio-orthogonal Staudinger ligation for the synthesis of CD dimers (M.D. Manouilidou, Y.G. Lazarou, I.M. Mavridis, K. Yannakopoulou, Beilstein J. Org. Chem. 2014, 10, 774 – 783). Despite self-inclusion, the ability of the dimers to encapsulate lipophilic molecules was both experimentally and theoretically demonstrated.
2) Molecular inclusion of the phosphorylated gemcitabine ((2,2′-difluorodeoxycytidine, dFdC)) into positively charged aminoalkyl-β-CDs leads to strong host-guest complexes, which may penetrate the membrane of cancer cells to properly deliver the phosphorylated nucleoside drug (V. Rodriguez-Ruiz, A. Maksimenko, G. Salzano, M. Lampropoulou, Y.G. Lazarou, V. Agostoni, P. Couvreur, R. Gref, K. Yannakopoulou, Sci. Rep. 2017, 7, 8353). The structure and stability of these complexes was theoretically examined at the PM7(COSMO) level of theory and the computational results were found to be in accordance with NMR data.