Antimicrobial and antibiofilm xerogel coatings
A. Xerogel coatings to bone implants prevent development of biofilm formation and severe osteomyelitis
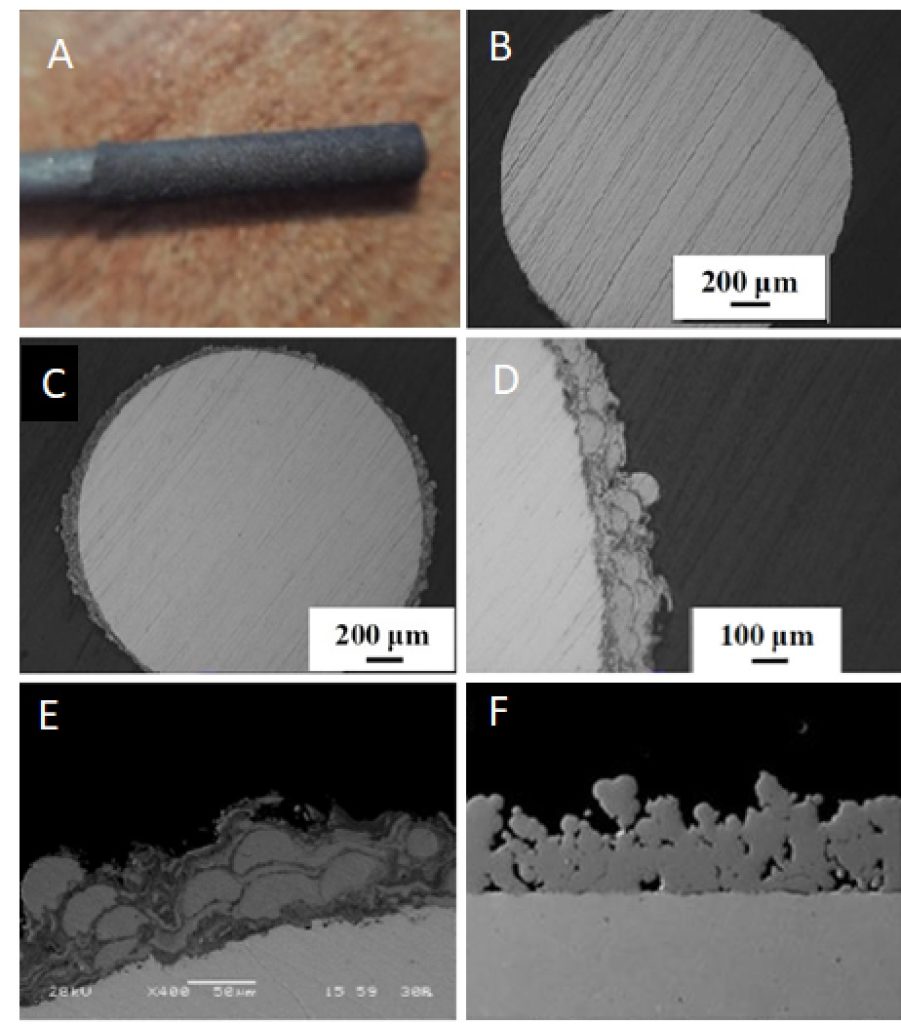
Chirurgical insertion risks and biofilm formation constitute major drawbacks to orthopaedical implants applications, since they may induce severe infections such as prosthetic joint infection (PJI) and implant associated osteomyelitis (IAO). The immobilization of antibacterial, antibiotic and/or antibiofilm compounds to metals is problematic. A pretreatment of model bone implants with metallic titanium microspheres via cold High Velocity Oxygen Fuel (Cold HVOF) thermal spray induces microporosity. Hydrogel formation reaction from orthosilisic acid and hyperbranched polyethyleneimine takes place into these artificial pores. The active ingredient may be introduced to the initial solution or for best results at the last stage to the dried xerogel. Multiple gel coatings and xerogel wettings by aqueous and/or organic solutions can be performed.1
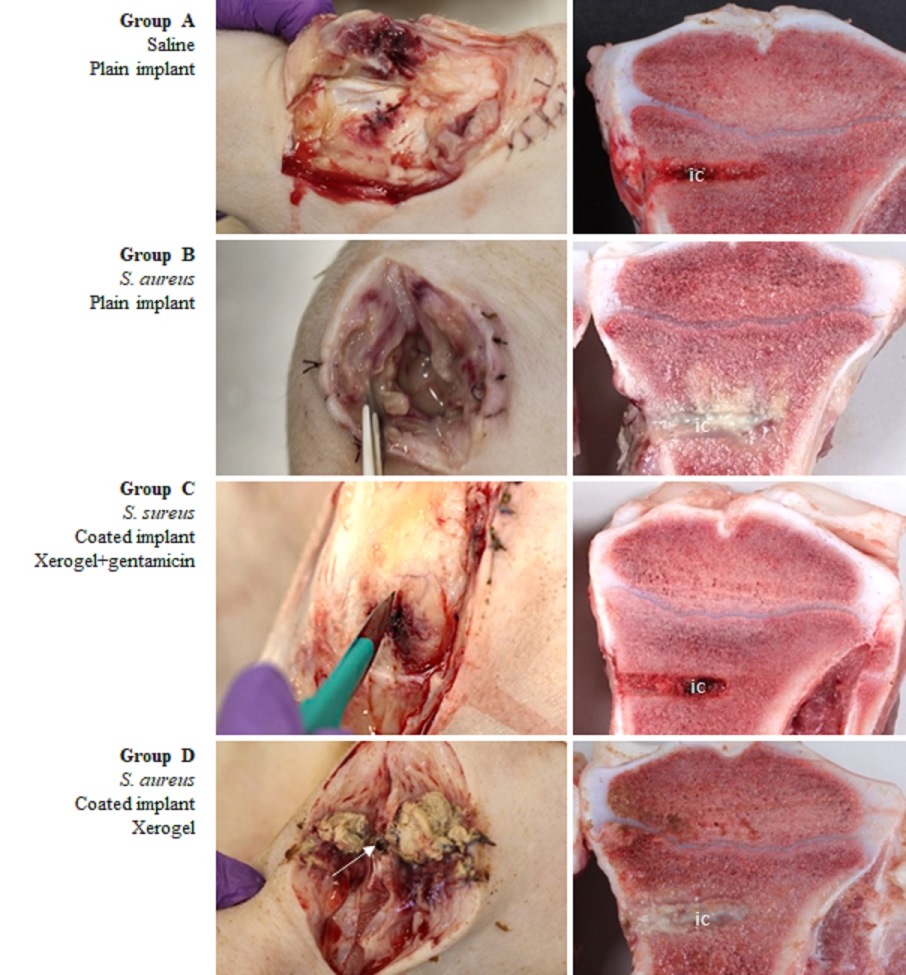
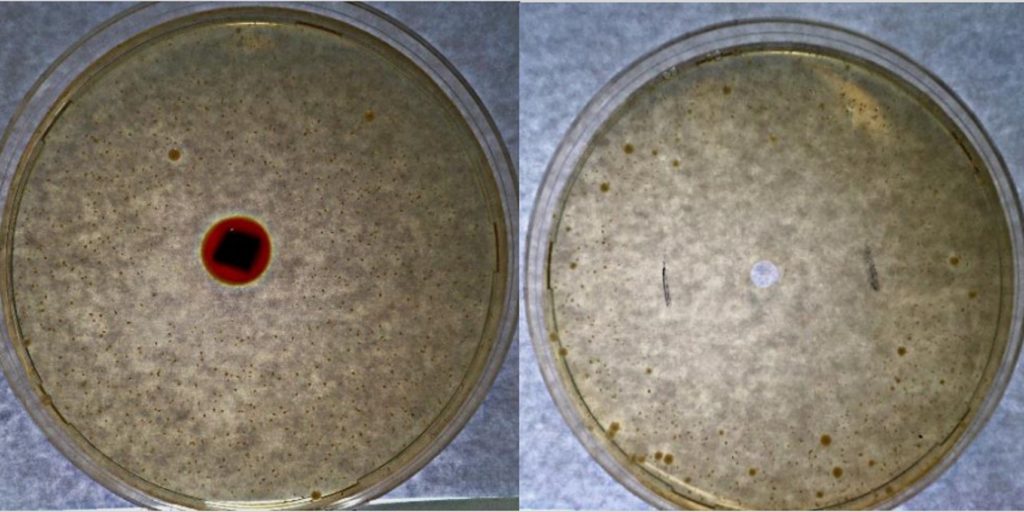
Chemical reactions and supramolecular interactions of antibiotics and antibiofilm compounds with the dendritic polymer are monitored spectroscopically and by DLC whereas their content was estimated by thermogravimetric analysis. Antibacterial and Antibiofilm tests are conducted against E. coli (HC1501), S. aureus (S207) and C. parapsilosis (SMI416) bacteria. Toxicity assessment are carried out using Caenorhabditis elegans model. The implants are being tested in vivo in a clinical representative large animal model (pigs inoculated with S. aureus S54F9 bacteria) by Macroscopic pathology, Serum analysis, Computed Tomography, Microbiology, Histology, Immunohistochemistry and Pharmacokinetics. They are exhibiting excellent results in preventing development of IAO and eradicating surgical contamination induced by a high virulent bacterium. This already established potential for prophylactic and therapeutic clinical applications may be extended to all pharmaceutical and osteo-synthetic compounds.
B. Hydrogels and xerogels active ingredient carriers from dendritic polymers and silica for use as additives in leathers and textiles
Hydrogels and xerogels consisting of silica, dendritic polymers and optionally metal nanoparticles are used as coatings to immobilize active ingredients to leathers or textiles. The chemical structure of dendritic polymers makes these inorganic-organic compounds ideal carriers for active ingredients such as antimicrobial, antibacterial, hypoallergenic, or compounds that enhance abrasion resistance, waterproofing, and facilitate water resistance. Gelation takes place within the pores of the solid support.2
Relevant Patents
1. M. Vardavoulias, E. Gkomoza, A. Tsetsekou, I. Kitsou, M. Arkas, M Papageorgiou, S. M. Soto González, Y. L. Cubillos, V. Cepas, H. E. Jensen, L. K. Jensen, S. Lopez-Ibanez, I. Gutierrez-del-Rio, C. J. Villar, F. Lombó, F. L. Ortiz, M. J. I. Valdés-Solís, Hydrogel and xerogelactive ingredient carriers made from dendritic polymers and silica for solid substrate coating applications. GR Application Number 20190100585.
2. M. Arkas, E. Nikoli, M. Douloudi, G. Kythreoti, L. Arvanitopoulos K. Arvanitopoulos, Hydrogel and xerogel active ingredient carriers made from dendritic polymers and silica for use as leather and textile additives. Greek Patent, eFiling Number 22-0003846572, 26/11/2020.