Toxic Compounds in Water
The presence of extremely toxic species in the aqueous environment, particularly lakes and groundwater, is addressed in this activity, in collaboration with the research groups of Prof. K. Kormas and Prof. C. Laspidou in the University of Thessaly in Volos, Greece.
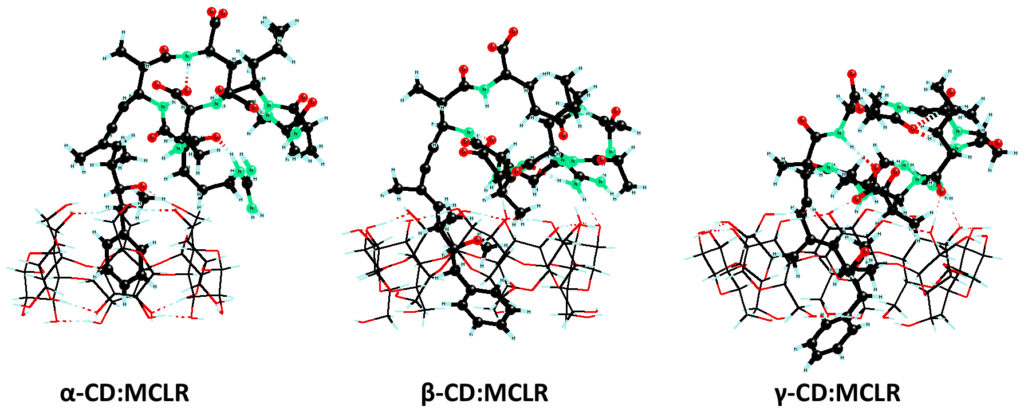
The removal of potentially hazardous cyanotoxins (CT) from water is investigated by considering cyclodextrins (CD) as efficient scavengers. The structures and energetics of CTs association with natural CDs were calculated at the semi-empirical PM7 level of theory incorporating an implicit solvent treatment (A.S. Archimandritis, T. Papadimitriou, K.A. Kormas, C.S. Laspidou, K. Yannakopoulou, Y.G. Lazarou, Sust. Chem. & Pharm. 2016, 3, 25-32). The thermodynamic parameters of the association reactions suggest that CDs may be considered as effective traps of microcystins and nodularins in aqueous solutions.
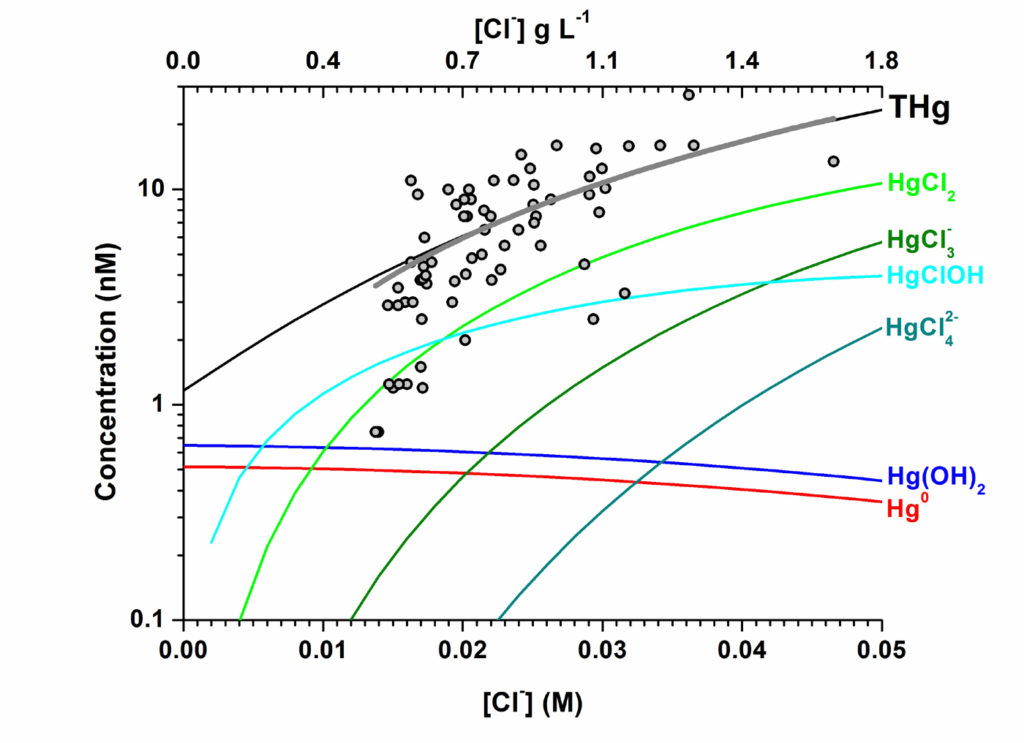
The seasonally variable presence of toxic mercury (Hg) species in coastal groundwater is also investigated, regarding the origins and speciation of Hg by using geochemical models as well as searching for suitable materials capable of Hg removal from water used for irrigation and drinking. Geochemical modeling suggests that the appearance of Hg in coastal groundwater is the result of seawater intrusion followed by mobilization of Hg(II) desorbed onto minerals represented most likely by ubiquitous hydrated ferric oxide. In addition, HgCln(2-n) (n = 2,3,4) coordination complexes constitute the primary Hg species resulting after seawater intrusion into the aquifer (A.E. Spyropoulou, Y.G. Lazarou, A.A. Sapalidis, C.S. Laspidou, Chemosphere 2022, 286, 131609).